
Biology 2e
2nd Edition
ISBN: 9781947172517
Author: Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher: OpenStax
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 3VCQ
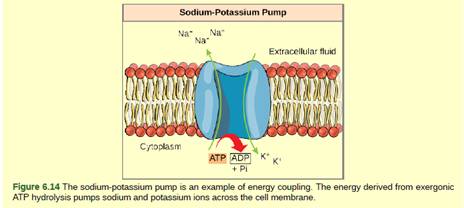
Figure 6.14 The hydrolysis of one ATP molecule releases 7.3 kcal/mol of energy (?G = -7.3 kcal/mol of energy). If it takes 2.1 kcal/mol of energy to move
one Na+ across the membrane (?G = +2.1 kcal/mol of energy), how many sodium ions could be moved by the hydrolysis of one ATP molecule?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
What type of energy helps drive the passive processes of diffusion, osmosis, and facilitated diffusion? (Pick ALL correct statements involved with this.) In other words what are all the TRUE statements regarding these processes?
Select one or more:
a.random motion which increases when temperature increases
b.ATP energy
c.random motion which increases when molecules have large molecular weight
d.random motion which increases when molecules concentration decreases
e.random motion which increases when molecules concentration increases
f.random motion which decreases when temperature decreases
g.random motion which increases when molecules have low molecular weight
What type of energy helps drive the passive processes of diffusion, osmosis, and facilitated diffusion? (Pick ALL correct statements involved with this.) In other words what are all the TRUE statements regarding these processes?
Select one or more:
a.
random motion which increases when molecules have low molecular weight
b.
random motion which decreases when temperature decreases
c.
random motion which increases when temperature increases
d.
random motion which increases when molecules concentration increases
e.
ATP energy
f.
random motion which increases when molecules have large molecular weight
g.
random motion which increases when molecules concentration decreases
if an 8.4-oz (250 mL) energy drink can has 27 grams of sugar, calculate the moles of ATP produced in the body when 1 oz of the drink is consumed assuming all the sugars in 1 oz of the drink are composed of only glucose.
Chapter 6 Solutions
Biology 2e
Ch. 6 - Figure 6.8 Look at each of the processes shown,...Ch. 6 - Figure 6.10 If no activation energy were required...Ch. 6 - Figure 6.14 The hydrolysis of one ATP molecule...Ch. 6 - Energy is stored long-term in the bonds of and...Ch. 6 - DNA replication involves unwinding two strands of...Ch. 6 - Consider a pendulum swinging. Which type(s) of...Ch. 6 - Which of the following comparisons or contrasts...Ch. 6 - Which of the following is the best way to judge...Ch. 6 - Which of the following is not an example of an...Ch. 6 - In each of the three systems, determine the state...
Ch. 6 - The energy released by the hydrolysis of ATP is...Ch. 6 - Which of the following molecules is likely to have...Ch. 6 - Which of the following is not true about enzymes...Ch. 6 - An allosteric inhibitor does which of the...Ch. 6 - Which of the following analogies best describes...Ch. 6 - Does physical exercise involve anabolic and/or...Ch. 6 - Name two different cellular functions that require...Ch. 6 - Explain in your own words the difference between a...Ch. 6 - Describe the position of the transition state on a...Ch. 6 - Imagine an elaborate ant farm with tunnels and...Ch. 6 - Energy transfers take place constantly in everyday...Ch. 6 - Do you think that the Ea for ATP hydrolysis is...Ch. 6 - With regard to enzymes, why are vitamins necessary...Ch. 6 - Explain in your own words how enzyme feedback...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Professional Application A 30,000-kg freight car is coasting at 0.850 m/s with negligible friction under a hopp...
College Physics
If someone at the other end of a room smokes a cigarette, you may breathe in some smoke. The movement of smoke ...
Campbell Essential Biology with Physiology (5th Edition)
1. What are the main organs of the skeletal system?
Human Anatomy & Physiology
What is anatomical position?
Human Anatomy & Physiology (2nd Edition)
One isomer of methamphetamine is the addictive illegal drug known as crank. Another isomer is a medicine for si...
Campbell Essential Biology (6th Edition) - standalone book
QUANTITATIVE Punnett Squares as Genetic Tools. The genetic characters of seed color (where Y is the allele for ...
Becker's World of the Cell (9th Edition)
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, biology and related others by exploring similar questions and additional content below.Similar questions
- How many net ATPs would be produced if the following fatty acid is completely oxidized intocarbon dioxides and water? 94.5 96.5 98 98.5 108arrow_forwardEach proton that moves across the membrane releases about 14 kJ/mol of energy. Given that ATP requires 30.5 kJ/mol of energy to form, how many protons cross the membrane per ATP synthesized? (Hint: can you have half a proton?)arrow_forwardThe relationship between ATP and ADP can be compared to the state of a battery. Which of the following statements best compares ATP,ADP and a battery?arrow_forward
- about ATP synthase [Cellular Respiration] Which of the following interactions provides the mechanical energy needed to promote ATP production? Non-polar interaction Salt bridge formation Polar interaction : Attractive Polar interaction : Repulsivearrow_forwardWhich one of the following statement is CORRECT for ATP? Select one: a. Divalent cation C12- stabilize ATP and blocks the driving force for association and make ATP unstable b. In pure water ATP Is very stable and high water concentration would only drive this reaction forward with the help of enzyme c. Phosphate bonds of ATP is "highly transferable" bonds. d. ATP is very unstable in salt solutions having near neutral pH therefore spontaneously dissociate into ADP and Parrow_forwardWhich of the following is NOT true of ATP? ATP is renewable ATP holds energy ATP in nonrenewable ATP is generated via Oxidative Phosphorylationarrow_forward
- CHOOSE THE CORRECT LETTER Which of the following conditions promote the synthesis of ATP through the electron transport chain?A.proton pump from outer mitochondrial membrane to inner mitochondrial membraneB. proton pump from matrix to inner mitochondrial membraneC. proton pump from inner mitochondrial membrane to outer mitochondrial membraneD. proton pump from inner mitochondrial membrane to matrixarrow_forwardWhich of the following statements concerning ATP is true? a. The free energy value for the hydrolysis of ATP is nearly the same for ADP. b. The free energy value for the hydrolysis of ATP is greater than that for ADP. c. ATP hydrolysis is more likely at pH 5 than at pH 7. d. One mole of glycerate-1,3-bisphosphate can phosphorylate one mole of AMP to yield ATP.arrow_forwardATP is used by cells for all of the following except ___ catabolic reactions intracellular movement (e.g. vesicular transport) transport solutes across the plasma membrane anabolic reactions muscle contractionarrow_forward
- Diatomic oxygen (O2) exhibits which of the following membrane transport movements? A. it is not able to cross the membrane by passive transport, because it is big, polar, and inorganic it is not able to cross the membrane by active transport, because it is big, polar, and inorganic it is able to cross the membrane by facilitated diffusion, because it is small, polar, and organic it is able to cross the membrane by active transport, because it is big, nonpolar, and organic it is able cross the membrane by simple diffusion, because it is small, nonpolar, and inorganicarrow_forwardWhich of the following statements describes the end step for the electron transport chain? A. H+ ions flow down the gradient to generate ATP B. electrons are transferred to NADH and FADH2 for chemiosmosis C. electrons are transferred to oxygen, causing it to split and take up H+ ions, which form water D. H+ are pumped across the inner membrane of mitochondria to establish an electrochemical gradientarrow_forwardHow many ATP can be generated from a single Phosphocreatine molecule?. a. 1 b. 2 c. 3 d. 36arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax
Biology 2eBiologyISBN:9781947172517Author:Matthew Douglas, Jung Choi, Mary Ann ClarkPublisher:OpenStax Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning
Biology Today and Tomorrow without Physiology (Mi...BiologyISBN:9781305117396Author:Cecie Starr, Christine Evers, Lisa StarrPublisher:Cengage Learning Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning
Human Physiology: From Cells to Systems (MindTap ...BiologyISBN:9781285866932Author:Lauralee SherwoodPublisher:Cengage Learning Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College
Concepts of BiologyBiologyISBN:9781938168116Author:Samantha Fowler, Rebecca Roush, James WisePublisher:OpenStax College

Biology 2e
Biology
ISBN:9781947172517
Author:Matthew Douglas, Jung Choi, Mary Ann Clark
Publisher:OpenStax

Biology Today and Tomorrow without Physiology (Mi...
Biology
ISBN:9781305117396
Author:Cecie Starr, Christine Evers, Lisa Starr
Publisher:Cengage Learning

Human Physiology: From Cells to Systems (MindTap ...
Biology
ISBN:9781285866932
Author:Lauralee Sherwood
Publisher:Cengage Learning

Concepts of Biology
Biology
ISBN:9781938168116
Author:Samantha Fowler, Rebecca Roush, James Wise
Publisher:OpenStax College

Anaerobic Respiration; Author: Bozeman Science;https://www.youtube.com/watch?v=cDC29iBxb3w;License: Standard YouTube License, CC-BY