
Concept explainers
(a)
Interpretation:
TheLewis structureof Acetaldehyde and a formal charge of each atom have to be written.
Concept Introduction:
A Lewis structure is also known as Lewis dot diagrams or electron dot structures. The bond between atoms and lone pairs of electrons that is present in the molecule. Lewis structure represents each atom and their position in structure using the chemical symbol. Excess electrons forms the lone pair are given by pair of dots, and are located next to the atom.
The formula for the formal charge can be written as
(a)
Explanation of Solution
The correct Lewis structure of the Acetaldehyde can be drawn as,
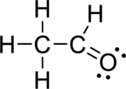
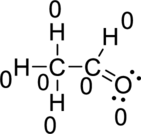
Draw a chart:
The total number of valence electron is
(b)
Interpretation:
The Lewis structure for
Concept Introduction:
Refer Part (a).
(b)
Explanation of Solution
There are three correct possible Lewis structure for
First structure:
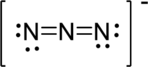
Draw a chart:
The total number of valence electron is
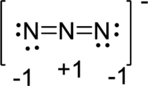
Second structure:
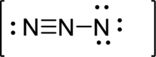
Draw a chart:
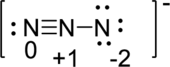
Third structure:
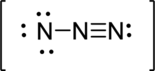
Draw a chart:
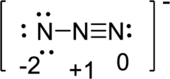
Therefore, the first of these three structures is the best one because the formal charge on atoms in that structure is lesser value
(c)
Interpretation:
The Lewis structure of
Concept Introduction:
Refer Part (a).
(c)
Explanation of Solution
The correct Lewis structure of the
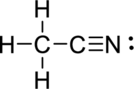
Draw a chart:
The total number of valence electron is
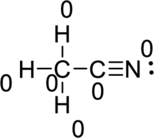
Hence, the formal charge of the
Want to see more full solutions like this?
Chapter 6 Solutions
EBK CHEMISTRY: THE MOLECULAR SCIENCE
- The thiocyanate ion , NCS- , has three possible Lewis structures.a. Draw these three structures and assign formal charges in each.b. Which Lewis structure is dominant?arrow_forwardDraw Lewis structures for these ions and show which atom (or atoms) in each bears the formal charge. Q.) OH-arrow_forwardThe cyanate ion, NCO-, has three possible Lewis structures.(a) Draw these three structures and assign formal charges ineach. (b) Which Lewis structure is dominant?arrow_forward
- Draw three resonance structures for carbonate ion, CO32-, and assign formal charges on all the atoms.arrow_forwardThe cyanate ion, NCO– , has three (3) possible Lewis structures. (a) Draw these three structures and assign formal charges in each. (b) Which Lewis structure is dominant?arrow_forwardDraw the Lewis structure with lowest formal charges, anddetermine the charge of each atom in (a) BF₄⁻; (b) ClNO.arrow_forward
- Write the correct Lewis structure and assign a formal charge to each atom in fulminate ion, CNO.arrow_forwardThree known isomers exist of N2CO, with the atoms in these sequences: NOCN; ONNC; and ONCN. Write resonance structures for each isomer and use formal charge to predict which isomer is the most stable.arrow_forwardBased on the concept of formal charge, what is the central atom in (a) HCN (do not include H as a possibility)? b) NOCI (Cl is always a terminal atom)?arrow_forward
- What is the formal charge on the anionic molecule (BrO3)-?arrow_forwardBased on average bond enthalpies, would you expect a photon capable ofdissociating a C¬Cl bond to have sufficient energy to dissociate a C¬Br bond?arrow_forwardWrite a Lewis structure for HC2─ and assign any formal charges to the correct atom.arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning

