
ORGANIC CHEMISTRY WILEYPLUS ACCESS>I<
4th Edition
ISBN: 9781119850151
Author: Klein
Publisher: WILEY
expand_more
expand_more
format_list_bulleted
Question
Chapter 6, Problem 58IP
Interpretation Introduction
Interpretation:
The compound 1 is prepared and studied to investigate the intramolecular elimination mechanism. The curved arrows are to be interpreted for the given reaction mechanism:
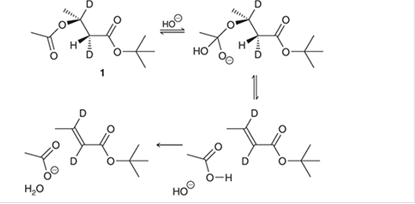
Concept introduction:
The chemical transformation can be shown by the arrow pushing method in which the curved arrows show the movement of electrons or the protons. In a
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Trimethyl Phosphite reacts with bromomethane to afford the product shown below via a two-step mechanism. Draw a detailed mechanism for this reaction using arrows to show electron flow (both mechanistic steps are SN2 reactions).
5. The target compound below (a potential ẞ-blocker drug) may be prepared using a sulfonium ylide (and
then a 2° amine) according to the retrosynthetic scheme shown below. Please perform the following:
(a) Draw the structure of a suitable ylide that would accomplish this task and (b) write a mechanism to
depict the formation and subsequent usage of the ylide (for the reaction in which the aldehyde is
converted into the epoxide)...so the mechanism does NOT have to include the amine + epoxide part of
the synthesis.
HO
NR2
1-8
potential class of
ẞ-blocker drugs
sulfonium ylide
H
Write a detailed mechanism for the following reaction.
KCN
HCN
Chapter 6 Solutions
ORGANIC CHEMISTRY WILEYPLUS ACCESS>I<
Ch. 6.1 - Prob. 1LTSCh. 6.1 - Prob. 1PTSCh. 6.1 - Prob. 2ATSCh. 6.2 - Prob. 3CCCh. 6.3 - Prob. 4CCCh. 6.3 - Prob. 5CCCh. 6.4 - Prob. 6CCCh. 6.6 - Prob. 7CCCh. 6.7 - Prob. 2LTSCh. 6.7 - Prob. 8PTS
Ch. 6.7 - Prob. 9PTSCh. 6.7 - Prob. 10ATSCh. 6.8 - Prob. 3LTSCh. 6.8 - Prob. 11PTSCh. 6.8 - Prob. 12ATSCh. 6.9 - Prob. 4LTSCh. 6.9 - Prob. 13PTSCh. 6.9 - Prob. 14ATSCh. 6.10 - Prob. 5LTSCh. 6.10 - Prob. 15PTSCh. 6.10 - Prob. 16ATSCh. 6.11 - Prob. 6LTSCh. 6.11 - Prob. 17PTSCh. 6.11 - Prob. 18ATSCh. 6 - Prob. 19PPCh. 6 - Prob. 20PPCh. 6 - Prob. 21PPCh. 6 - Prob. 22PPCh. 6 - Prob. 24PPCh. 6 - Prob. 25PPCh. 6 - Prob. 26PPCh. 6 - Prob. 27PPCh. 6 - Prob. 28PPCh. 6 - Prob. 29PPCh. 6 - Prob. 30PPCh. 6 - Prob. 31PPCh. 6 - Prob. 32PPCh. 6 - Prob. 33PPCh. 6 - Prob. 34PPCh. 6 - Prob. 35PPCh. 6 - Prob. 36PPCh. 6 - Prob. 37PPCh. 6 - Prob. 38PPCh. 6 - Prob. 39PPCh. 6 - Prob. 40PPCh. 6 - Prob. 41PPCh. 6 - Prob. 43ASPCh. 6 - Prob. 44ASPCh. 6 - Prob. 45ASPCh. 6 - Prob. 46ASPCh. 6 - Prob. 47ASPCh. 6 - Prob. 48ASPCh. 6 - Prob. 49ASPCh. 6 - Prob. 50IPCh. 6 - Prob. 51IPCh. 6 - Prob. 52IPCh. 6 - Prob. 53IPCh. 6 - Prob. 54IPCh. 6 - Prob. 55IPCh. 6 - Prob. 56IPCh. 6 - Prob. 57IPCh. 6 - Prob. 58IPCh. 6 - Prob. 59IPCh. 6 - Prob. 60IPCh. 6 - Prob. 61IPCh. 6 - Prob. 62CPCh. 6 - Prob. 64CP
Knowledge Booster
Similar questions
- Use the reaction given below to answer the following questions. A CH₂OH B H₂O+ C + CH3NH3* D a) What are the approximate pKas of compounds A and B? b) The reaction given above is irreversible. Give a detailed explanation of the reaction and reaction mechanism. Your explanation should indicate your knowledge of the reactivity of carboxylic acid derivatives.arrow_forwardPropose a detailed mechanism for the transformations presented. represent the Intermediate structure A.arrow_forwardTreatment of a cyclic ketone with diazomethane is a method for accomplishing a ring-expansion reaction. The reaction involves the initial nucleophilic attack by diazomethane on the carbonyl carbon to form a tetrahedral intermediate. Collapse of this intermediate is accompanied by bond migration and loss of N,. For example, treatment of cyclohexanone with diazomethane yields cycloheptanone. CH2N2, ether + N2 Draw the structure of the organic product(s) of the ring expansion of this compound: • You do not have to consider stereochemistry. • You do not have to explicitly draw H atoms. • Do not include lone pairs in your answer. They will not be considered in the grading. • Draw one structure per sketcher Add additional ketchers usina the dron down menu in the bottom right co Previous Nextarrow_forward
- b) Synthesize the product on the right using any two organohalides of your choice (R-X, R'-X) with six carbon atoms or less in their structures. Describe the preparation of any organometallic species and cross-coupling conditions R-X R₁-Xarrow_forwardPlease describe the mechanism in words for the reaction below. DO NOT provide a mechainism.arrow_forwardPredict the intermediate (A) and Reagent (B) in the reaction sequence below.arrow_forward
- Provide the complete mechanism using Curved Arrow Formalism for the reaction shown below. State how many signals would be visualized in the 13C NMR spectrum for the product and give any pertinent IR data. 1. КОН, Н2О, heat, CH3(CH2),CN 2. HО → CH3(CH2),COHarrow_forwardProvide a detailed mechanism that explains the formation of the reaction shown below:arrow_forwardgas, Cl¬CH2CH2¬S¬CH2CH2¬Cl, was used as a poisonous chemical agentin World War I. Mustard gas is much more toxic than a typical primary alkyl chloride. Itstoxicity stems from its ability to alkylate amino groups on important metabolic enzymes,rendering the enzymes inactive.(a) Propose a mechanism to explain why mustard gas is an exceptionally potent alkylatingagent.(b) Bleach (sodium hypochlorite, NaOCl, a strong oxidizing agent) neutralizes and inactivates mustard gas. Bleach is also effective on organic stains because it oxidizes coloredcompounds to colorless compounds. Propose products that might be formed by thereaction of mustard gas with bleach.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
