
Concept explainers
Drawing Structural and Condensed Structural Formulas
Give the molecular formula, and draw the structural formula and condensed structural formula for the
Interpretation:
The molecular formula, structural formula and condensed structural formula are to be represented for the alkane with
Concept Introduction:
The organic compounds which consist of only carbon and hydrogen atoms are called hydrocarbons. The atoms in hydrocarbons are linked by single, double, or triple bonds.
The graphic representation of a chemical compound is called its structural formula. It represents the arrangement of atoms.
In a condensed structural formula, the bond between atoms in a compound is represented by the absence of a line.
The chemical formula that represents the total number of atoms arranged in a molecule is known as the molecular formula.
Answer to Problem 6.1YT
Solution: Molecular formula for an alkane is given as:
The structural formula for an alkane contains
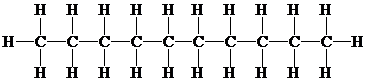
The condensed structural formula for an alkane has
Or
Explanation of Solution
The molecular formula for an alkane with
The structural formula for an alkane compound which contains
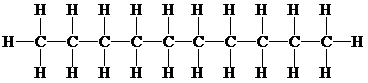
The condensed structural formula for a pentane consists of
Or
Molecular formula, the structural formula and the condensed structural formula for an alkane has
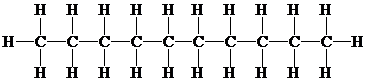
and
Or
Want to see more full solutions like this?
Chapter 6 Solutions
Bundle: Chemistry In Focus: A Molecular View Of Our World, 7th + Owlv2 With Mindtap Reader, 1 Term (6 Months) Printed Access Card
- Use the generic formula for alkanes (CnH2n+2) to derive molecular and condensed structural formulas for: a. Propane, 3 carbon atoms b. Octane, 8 carbon atoms c. Butane, 4 carbon atomsarrow_forwardIs the general formula of a cycloalkanes the same as the general formula of an alkane, CnH2n+2? Draw any structural diagram to illustrate your answer.arrow_forward

 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage LearningChemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning





