
Concept explainers
(a)
Interpretation:
Whether the statement “In some cases, constitutional isomers are chiral” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
The compounds which have same molecular formula but different connectivity of atoms are known as constitutional isomers. Chiral compounds are those compounds which contain an asymmetric carbon atom. Chiral molecules are optically active molecules.
Answer to Problem 6.34AP
The statement “In some cases, constitutional isomers are chiral” is true.
Explanation of Solution
There are many examples where constitutional isomers are chiral. In case of
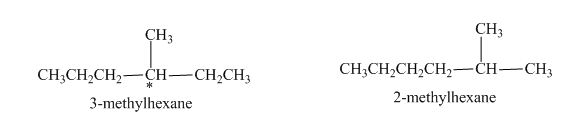
Figure 1
The statement “In some cases, constitutional isomers are chiral” is found to be true.
(b)
Interpretation:
Whether the statement “In every case, a pair of enantiomers have a mirror-image relationship” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
The pair of stereoisomer which is mirror images of each other is known as enantiomers. Enantiomers are non-congruent mirror images. If the molecules are placed on top of each other they will not give same molecule.
Answer to Problem 6.34AP
The statement “In every case, a pair of enantiomers have a mirror-image relationship” is true.
Explanation of Solution
Enantiomers are defined as non-congruent mirror image. The enantiomers pair in every case will have mirror image relationship. Therefore, the statement “In every case, a pair of enantiomers have a mirror-image relationship” is true.
The statement “In every case, a pair of enantiomers have a mirror-image relationship” is found to be true.
(c)
Interpretation:
Whether the statement “Mirror-image molecules are in all cases enantiomers” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
The pair of stereoisomer which is mirror images of each other is known as enantiomers. Enantiomers are non-congruent mirror images. If the molecules are placed on top of each other they will not give same molecule.
Answer to Problem 6.34AP
The statement “Mirror-image molecules are in all cases enantiomers” is false.
Explanation of Solution
The molecules which contain asymmetric carbon atom are chiral molecule. Pair of chiral molecules which are non-congruent mirror image of each other are enantiomers. Achiral molecules which are mirror image of each other are not enantiomers. Therefore, the statement “Mirror-image molecules are in all cases enantiomers” is false.
The given statement “Mirror-image molecules are in all cases enantiomers” is found to be false.
(d)
Interpretation:
Whether the statement “If a compound has an enantiomer, it must be chiral” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
The pair of stereoisomer which is mirror images of each other is known as enantiomers. Enantiomers are non-congruent mirror images. If the molecules are placed on top of each other they will not give same molecule.
Answer to Problem 6.34AP
The statement “If a compound has an enantiomer, it must be chiral” is true.
Explanation of Solution
The molecules which contain asymmetric carbon atom are chiral molecule. Pair of chiral molecules which are non-congruent mirror image of each other are enantiomers. Therefore, the given statement is true.
The given statement “If a compound has an enantiomer, it must be chiral” is found to be true.
(e)
Interpretation:
Whether the statement “Every chiral compound has a diastereomer” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
Stereoisomers which are non-superimposable and not mirror image are known as diastereomers. The compound must contain two or more than two stereocentre. Diastereomer are non identical stereoisomers.
Answer to Problem 6.34AP
The statement “Every chiral compound has a diastereomer” is false.
Explanation of Solution
Diastereomers are compound which are non-congruent and not mirror images of each other. The compound must contain two or more than two stereocentre to form diastereomer. Chiral molecules can also have only one stereocentre. Therefore, given statement is false.
The given statement “Every chiral compound has a diastereomer” is found to be false.
(f)
Interpretation:
Whether the statement “If a compound has a diastereomer, it must be chiral” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
Stereoisomers which are non-superimposable and not mirror image are known as diastereomer. The compound must contain two or more than two stereocentre. Diastereomer are non identical stereoisomers.
Answer to Problem 6.34AP
The statement “If a compound has a diastereomer, it must be chiral” is false.
Explanation of Solution
Diastereomers are not always chiral. A meso compound is a diastereomer, but it is achiral due to internal plane of symmetry. Therefore, given statement is false. An example is shown below.
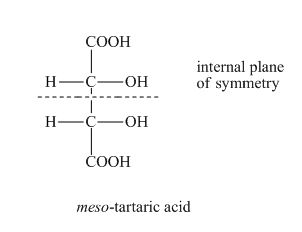
Figure 2
The statement “If a compound has a diastereomer, it must be chiral” is found to be false.
(g)
Interpretation:
Whether the statement “Every molecule containing one or more asymmetric carbons is chiral” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
Chiral compounds are those compounds which contain an asymmetric carbon atom. Chiral molecules are optically active molecules. Chiral molecule can rotate the plane of polarized light.
Answer to Problem 6.34AP
The statement “Every molecule containing one or more asymmetric carbons is chiral” is false.
Explanation of Solution
The meso compounds contain two asymmetric carbon centres. Meso compounds are achiral, due to the presence of internal plane of symmetry. Therefore the given statement is false.
The given statement “Every molecule containing one or more asymmetric carbons is chiral” is found to be false.
(h)
Interpretation:
Whether the statement “Any molecule containing a stereocenter must be chiral” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
Chiral compounds are those compounds which contain an asymmetric carbon atom. Chiral molecules are optically active molecules. Chiral molecule can rotate the plane of polarized light.
Answer to Problem 6.34AP
The statement “Any molecule containing a stereocenter must be chiral” is false.
Explanation of Solution
The
The given statement “Any molecule containing a stereocenter must be chiral” is found to be false.
(i)
Interpretation:
Whether the statement “Any molecule with a stereocenter must have a stereoisomer” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
Chiral compounds are those compounds which contain an asymmetric carbon atom. Chiral molecules are optically active molecules. Chiral molecule can rotate the plane of polarized light. Stereocentre can be an atom, bond, or any point in molecule at which interchange of two groups form a stereoisomer.
Answer to Problem 6.34AP
The statement “Any molecule with a stereocenter must have a stereoisomer” is true.
Explanation of Solution
Stereocentre can be an atom, bond, or any point in molecule at which interchange of two groups form a stereoisomer. If a molecule contains a stereocenter, therefore it will contain an asymmetric carbon atom. This means the molecule has a stereoisomer. Therefore, the given statement is true.
The statement “Any molecule with a stereocenter must have a stereoisomer” is found to be true.
(j)
Interpretation:
Whether the statement “Some diastereomers have a mirror-image relationship” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
Stereoisomers which are non-superimposable and not mirror image are known as diastereomers. The compound must contain two or more than two stereocentre. Diastereomer are non identical stereoisomers.
Answer to Problem 6.34AP
The given statement “Some diastereomers have a mirror-image relationship” is false.
Explanation of Solution
Diastereomers are stereoisomers which are not identical and not mirror image of each other. Diastereomers will never have mirror image relationship. Therefore, the given statement is false.
The given statement “Some diastereomers have a mirror-image relationship” is found to be false.
(k)
Interpretation:
Whether the statement “Some chiral compounds are optically inactive” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
Chiral compounds are those compounds which contain an asymmetric carbon atom. Chiral molecules are optically active molecules. Chiral molecule can rotate the plane of polarized light.
Answer to Problem 6.34AP
The statement “Some chiral compounds are optically inactive” is false.
Explanation of Solution
Chiral compounds are those compounds which contain an asymmetric carbon atom. The chiral compounds are always optically active compounds. The optically inactive compounds are known as achiral compounds. Therefore, the given statement is false.
The given statement “Some chiral compounds are optically inactive” is found to be false.
(l)
Interpretation:
Whether the statement “Any chiral compound with a single asymmetric carbon must have a positive optical rotation if the compound has the R configuration” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
Enantiomers rotate the plane of polarized light in opposite direction with equal amount. If compound rotates plane of polarized light in right hand direction, it is dextrorotatory. If compound rotates plane of polarized light in left hand direction, it is levorotatory.
Answer to Problem 6.34AP
The statement “Any chiral compound with a single asymmetric carbon must have a positive optical rotation if the compound has the R configuration” is false.
Explanation of Solution
The compound having R configuration can rotate the plane of polarized light in any direction. The compound will have both dextrorotatory and levorotatory molecules. Therefore, the given statement is false.
The given statement “Any chiral compound with a single asymmetric carbon must have a positive optical rotation if the compound has the R configuration” is found to be false.
(m)
Interpretation:
Whether the statement “A structure is chiral if it has no plane of symmetry” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
A molecule is said to be chiral, if it does not contain any symmetry element. There are many symmetry elements like plane of symmetry, centre of symmetry and axis of symmetry. The absence of all the symmetry elements is essential for the structure to be chiral.
Answer to Problem 6.34AP
The statement “A structure is chiral if it has no plane of symmetry” is false.
Explanation of Solution
For a structure to be chiral, it must not contain any symmetry element. Only plane of symmetry is not considered, centre of symmetry is also considered. If the molecule does not contain any symmetry element only then it will be chiral. Therefore, the given statement is false.
The given statement “A structure is chiral if it has no plane of symmetry” is found to be false.
(n)
Interpretation:
Whether the statement “All chiral molecules have no plane of symmetry” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
A molecule is said to be chiral, if it does not contain any symmetry element. There are many symmetry elements like plane of symmetry, centre of symmetry and axis of symmetry. The absence of all the symmetry elements is essential for the structure to be chiral.
Answer to Problem 6.34AP
The statement “All chiral molecules have no plane of symmetry” is true.
Explanation of Solution
The chirality of molecule depends on the absence of all the symmetry elements. All chiral molecules have no plane of symmetry, no centre of symmetry and no axis of symmetry. Therefore, the given statement is true.
The given statement “All chiral molecules have no plane of symmetry” is found to be true.
(o)
Interpretation:
Whether the statement “All asymmetric carbons are stereocenters” is true or false is to be predicted. If it is false then the explanation is to be stated.
Concept Introduction:
The compounds which have same molecular formula but different connectivity of atoms are known as constitutional isomers. Chiral compounds are those compounds which contain an asymmetric carbon atom. Chiral molecules are optically active molecules.
Answer to Problem 6.34AP
The statement “All asymmetric carbons are stereocenters” is true.
Explanation of Solution
The stereo center can be a point, atom, bond in a molecule which makes the molecule asymmetric. All asymmetric carbons are stereocentres. Therefore, given statement is true.
The given statement “All asymmetric carbons are stereocenters” is found to be true.
Want to see more full solutions like this?
Chapter 6 Solutions
ORGANIC CHEMISTRY (LL)+ SAPLING ACC >BI
- Which of the following molecules exhibits chirality?arrow_forwardHow many chirality centers are there in the following molecule?arrow_forwardIf molecules A and B are isomers of each other, then what kinds of isomers could they be (i.e., enantiomers, diastereomers, or constitutional isomers) under each of the following conditions?(a) Both molecules have the same IHD.(b) Molecule A has a ring but molecule B does not.(c) Molecules A and B contain different functional groups.(d) Molecules A and B share exactly the same functional groups.(e) Molecule A has a plane of symmetry but molecule B does not.arrow_forward
- are these enantiomers, complete different, constitutional isomers or diastereomers? and why ?arrow_forwardHow many chirality centers are there in the molecule shown below? How many stereoisomers are possible?arrow_forwardProvide an example of a molecule that has 4 chirality centres, but does not have 16 stereoisomers. Why doesn’t this molecule have 2n stereoisomers? Explain.arrow_forward
- could you explain the concept of chirality in organic chemistry? I understand what an enantiomer is, also diastereoisomers and meso.....but chirality is somehow not registeringarrow_forwardIdentify chiral center and assign configuration (R or S) of each one in the compound.arrow_forwardThe following compound has only one asymmetric center. Why then does it have four stereoisomers?arrow_forward
- Give the stereochemical designation for each of the chiral centers pointed out by arrowsarrow_forwardFind an example of a chiral molecule in biology whose enantiomers have markedly different functions. Where is the chiral center located, and how can it be identified? Find and include an image of its molecular structure. What function does the active enantiomer play biologically, and how is this function hindered or changed with the other enantiomer? Are there any synthetic considerations for this molecule?arrow_forwardIs it possible for a meso compound to contain three chiral centers? Why or why not?arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
 Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole
Macroscale and Microscale Organic ExperimentsChemistryISBN:9781305577190Author:Kenneth L. Williamson, Katherine M. MastersPublisher:Brooks Cole


