
(a)
Interpretation:
Calculate values of
Concept Introduction:
The Gibbs free energy is calculated as:
(a)
Answer to Problem 6.46P
Explanation of Solution
Given information:
It is given that pressure is
From steam tables of saturated steam in Appendix E, table E.1
At pressure
Since, pressure
From linear interpolation, if
Temperature corresponding to
Enthalpy of saturated liquid and entropy of saturated liquid and vapor is
And,
Entropy of saturated liquid and entropy of saturated liquid and vapor is
And,
So,
And
Both values of
(b)
Interpretation:
Calculate values for
Concept Introduction:
The change in enthalpy and entropy in ideal gas is defined as:
And
(b)
Answer to Problem 6.46P
Explanation of Solution
From subpart (a), values of enthalpies of saturated vapor and liquid as well as values of entropies of saturated vapor and liquid are:
And temperature is:
So,
And
Both values of
(c)
Interpretation:
Calculate values for
Concept Introduction:
The residual properties
The residual properties
The residual properties
(c)
Answer to Problem 6.46P
Explanation of Solution
At
So,
Now, for the hypothetical ideal gas values of volume, enthalpy and entropy at same temperature and pressure, it cannot find using steam table because steam tables only give values of real gases not ideal gases. However, we can make an approximation of low pressure in real gases at the same temperature to convert real gas into ideal one. So, we are considering low pressure or
Since,
Hence from linear interpolation, at
But we want values of volume, enthalpy and entropy of ideal gas at
And
Enthalpy of ideal gas is defined as
Which is not the function of pressure, so enthalpy of ideal gas at
Now,
For entropy we know that
Since temperature s constant so first term at right hand side will be zero and hence,
So,
Residual properties are:
And
And
Now calculations of residual properties of volume, enthalpy and entropy for saturated vapor from generalized correlations are given below,
....(1a)
Where,
....(1b)
....(2a)
Where,
....(2b)
And
....(3a)
Where
....(3b)
Properties of pure species of steam are given in Table B.1 Appendix B as water,
So,
And
For differentiative terms in equation (2b) and (3b),
And,
For residual volume calculations, From equation (1b)
So, from equation (1a)
For residual enthalpy calculations, From equation (2b)
So, from equation (2a)
For residual entropy calculations, from equation (3b)
So, from equation (3a)
Results do not agree but approximately they do agree.
(d)
Interpretation:
Calculate values for
Concept Introduction:
First draw a graph between
The Clapeyron equation is:
(d)
Answer to Problem 6.46P
Explanation of Solution
From saturated steam table in Appendix E, table E.2
Now, draw a graph between
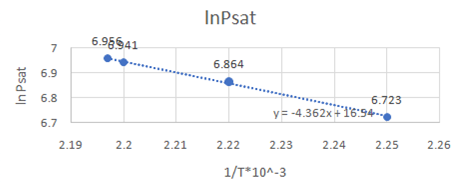
Slope of the graph from equation of graph is
Or,
So,
Also,
Volume of saturated liquid and entropy of saturated liquid and vapor at pressure
Since, pressure
And,
So,
Now from Clapeyron equation,
The value of
So, the results approximately match with each other.
Want to see more full solutions like this?
Chapter 6 Solutions
GEN, ORG & BIOL CHEM: CUSTOM SSC
 Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education
Introduction to Chemical Engineering Thermodynami...Chemical EngineeringISBN:9781259696527Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark SwihartPublisher:McGraw-Hill Education Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...Chemical EngineeringISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
Elements of Chemical Reaction Engineering (5th Ed...Chemical EngineeringISBN:9780133887518Author:H. Scott FoglerPublisher:Prentice Hall
 Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning
Industrial Plastics: Theory and ApplicationsChemical EngineeringISBN:9781285061238Author:Lokensgard, ErikPublisher:Delmar Cengage Learning Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The
Unit Operations of Chemical EngineeringChemical EngineeringISBN:9780072848236Author:Warren McCabe, Julian C. Smith, Peter HarriottPublisher:McGraw-Hill Companies, The





