
(a)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanamine (
Concept introduction:
Leveling effect refers to the effect of a solvent on the properties of acids and bases. For an acid-base reaction, the strength of the strong acid is limited or leveled by the basicity of the solvent. Similarly, the strength of the strong base is leveled by the acidity of the solvent. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.46P
With respect to the leveling effect, ethanamine is a suitable solvent for a reaction involving the chloride ion
Explanation of Solution
The reaction of chloride ion
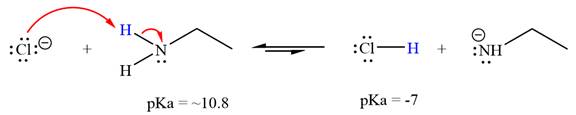
Hydrochloric acid,
The solvent effect on the reactant is determined with respect to the leveling effect.
(b)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanamine (
Concept introduction:
Leveling effect refers to the effect of a solvent on the properties of acids and bases. For an acid-base reaction, the strength of the strong acid is limited or leveled by the basicity of the solvent. Similarly, the strength of the strong base is leveled by the acidity of the solvent. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.46P
With respect to the leveling effect, ethanamine is a not suitable solvent for a reaction involving
Explanation of Solution
The reaction of
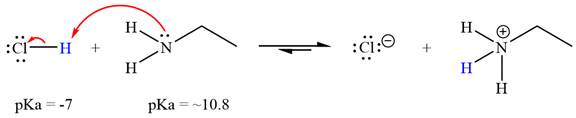
Hydrochloric acid,
The solvent effect on the reactant is determined with respect to the leveling effect.
(c)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanamine (
Concept introduction:
Leveling effect refers to the effect of a solvent on the properties of acids and bases. For an acid-base reaction, the strength of the strong acid is limited or leveled by the basicity of the solvent. Similarly, the strength of the strong base is leveled by the acidity of the solvent. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.46P
With respect to the leveling effect, ethanamine is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of

Ethanamine,
The solvent effect on the reactant is determined with respect to the leveling effect.
(d)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanamine (
Concept introduction:
Leveling effect refers to the effect of a solvent on the properties of acids and bases. For an acid-base reaction, the strength of the strong acid is limited or leveled by the basicity of the solvent. Similarly, the strength of the strong base is leveled by the acidity of the solvent. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.46P
With respect to the leveling effect, ethanamine is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of
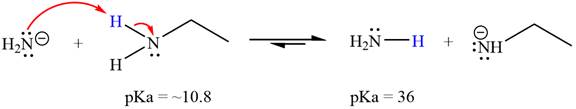
Ethanamine,
The solvent effect on the reactant is determined with respect to the leveling effect.
(e)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanamine (
Concept introduction:
Leveling effect refers to the effect of a solvent on the properties of acids and bases. For an acid-base reaction, the strength of the strong acid is limited or leveled by the basicity of the solvent. Similarly, the strength of the strong base is leveled by the acidity of the solvent. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.46P
With respect to the leveling effect, ethanamine is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of
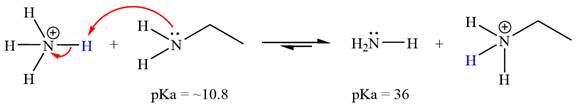
Ethanamine,
The solvent effect on the reactant is determined with respect to the leveling effect.
(f)
Interpretation:
It is to be determined whether the given reactant is suitable for a reaction involving ethanamine ( ) as a solvent with respect to leveling effect.
Concept introduction:
Leveling effect refers to the effect of a solvent on the properties of acids and bases. For an acid-base reaction, the strength of the strong acid is limited or leveled by the basicity of the solvent. Similarly, the strength of the strong base is leveled by the acidity of the solvent. With respect to the leveling effect, a solvent is unsuitable for a particular reactant R if R is a stronger acid that the solvent’s conjugate acid (i.e., R has the lower pKa) or if R is a stronger base than the solvent’s conjugate base (i.e., the conjugate acid of R has a higher pKa than the solvent).
Answer to Problem 6.46P
With respect to the leveling effect, ethanamine is not a suitable solvent for a reaction involving
Explanation of Solution
The reaction of hydroxide ion
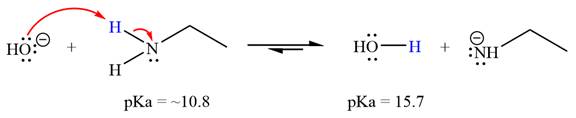
Ethanamine,
The solvent effect on the reactant is determined with respect to the leveling effect.
Want to see more full solutions like this?
Chapter 6 Solutions
ORG.CHEM W/TEXT+SOLU.MANUAL
- What can you conclude regarding the difference in degree of acidity and alkalinity between organic and inorganic compounds? ( sample substances are dilute hcl, dilute acetic acid, dilute nh4oh and aniline).arrow_forwardExplain why the hydrolysis of [Co(NH3)5Cl]2+ in the presence of base muchfaster than that of [Co(py)5Cl]2+? Illustrate your answer with suitable diagrams.arrow_forwardin the reaction, PrBr with NH3 gives a mixture of products. Why is an excess of NH3 required to give propylamine, PrNH2 as the major product?arrow_forward
- What happens to emission intensity when KI is added to a fluorescein solution and why?arrow_forwardprepare a flow sheet for the preparation of benzoic acid from a) bromobenzene and magnesium and b) phenylmagnesium bromide and carbon dioxide. using your knowledge of the physical properties of the solvents, reactants, and products, show how the products can be purified, indicate which layer should contain the product in the liquid/liquid extraction steps.arrow_forwardPropose a reasonable Phase I and a Phase II metabolite for the followingarrow_forward
- You are in a laboratory that has access to all chemical reagents. How would you synthesize compounds that contain the RnF5(+) and the RnF5(-) ions? Hint: your first step will be the reaction of two elements!arrow_forwardWhich of these structures reacts with Br2 in the dark?arrow_forwardSuggest a reason why the NH2 group depicted in the reaction scheme is the one used to form the semicarbazone and not the other NH2 group.arrow_forward
- The reaction for the magnesium cation with 8-hydroxyquinoline is carried out in the presence of: A) Nitric acid B) Sodium hydroxide C) A solution of ammonia and ammonium chloride D) Sulfuric acid E) Sodium acetatearrow_forwardN-H bonds in ammonia NH_3NH3 produce an IR absorbance at 3335 cm^-1. P-H bonds in phosphine PH_3PH3 produce an IR absorbance at 2327 ccm−1. Explain the difference in a single sentencearrow_forwardHow does the Hinsberg test allow for the distinction of primary, secondary and tertiary amines? Show this by means of a diagram and by reaction mechanism.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
