
ORGANIC CHEMISTRY PKG *FULL YEAR* >CI<
8th Edition
ISBN: 9781256779612
Author: WADE + BRUICE
Publisher: PEARSON C
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 6, Problem 72P
The second-order rate constant (in units of M–1s–1) for acid-catalyzed hydration at 25 °C is given (or each of the following
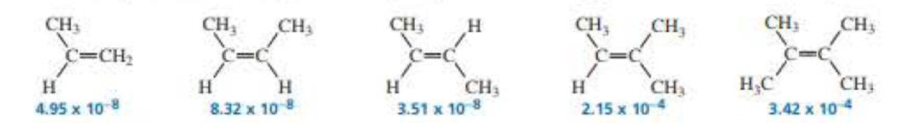
- a. Calculate the relative rotes of hydration of the alkenes. (Hint: Divide each rate constant by the smallest rate constant of the series: 3.51 × 10–8.)
- b. Why does (Z)-2-butene react faster than (E)-2-butene?
- c. Why does 2-methyl-2-butene react faster than (Z)-2-butene?
- d. Why does 2.3-dimethyl-2-butene react faster than 2-melhyl-2·butene?
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
write the reaction of the action of methyl-2, bromo-2-propane with hot KOH solution.
a) Name the formed product
b) Explain why the reaction takes place according to the SNI mechanism
For the base-catalysed hydrolysis of 3-bromo-3-methylhexane (i.e. reaction with the nucleophile OH-):
State whether the reaction is likely to proceed by an SN1 or SN2 mechanism, and explain why
give the likely rate law and explain why
1. What is the function of CH2Cl2 in the bromination reactions? Why can it fulfill this role?2. In not more than three (3) sentences, explain why terminal alkynes are acidic.3. What impurities are removed when acetylene gas is made to pass through an acidified solution of CuSO4?4. Explain the difference in the rate of free-radical bromination reactions of toluene and cyclohexane.5. Give the reagent or chemical test that would differentiate the following pairs o fcompounds. Provide only the reagents or chemical tests discussed in the module. Write chemical equations for the reactions involved.
a. benzene and ethylbenzeneb. 1-butyne and 2-butynec. 2-methylpentane and 2-methyl-2-pentened. toluene and 1-methylcyclohexene
Chapter 6 Solutions
ORGANIC CHEMISTRY PKG *FULL YEAR* >CI<
Ch. 6.1 - Draw the mechanism for the reaction of cyclohexene...Ch. 6.2 - a. How many bond orbitals are avilable for...Ch. 6.2 - Prob. 3PCh. 6.2 - Prob. 4PCh. 6.3 - Prob. 5PCh. 6.4 - Prob. 6PCh. 6.4 - Prob. 7PCh. 6.4 - What alkene should be used to synthesize each of...Ch. 6.5 - The pKa of a protonated alcohol is about 2.5, and...Ch. 6.5 - Prob. 10P
Ch. 6.5 - Prob. 11PCh. 6.6 - a. What is the major product or each or the...Ch. 6.6 - Prob. 14PCh. 6.6 - Prob. 15PCh. 6.7 - What is the major product obtained from the...Ch. 6.8 - Which is more highly regionselective: reaction of...Ch. 6.8 - Prob. 19PCh. 6.9 - What will be the product of the preceding reaction...Ch. 6.9 - Prob. 21PCh. 6.9 - Prob. 22PCh. 6.9 - Prob. 23PCh. 6.9 - What is the product of the addition of 1Cl to...Ch. 6.9 - What will be the major product obtained from the...Ch. 6.9 - Propose a mechanism for the following reaction:Ch. 6.10 - Draw structures for the following: a. 24...Ch. 6.10 - What alkene would you treat with a peroxyacid in...Ch. 6.11 - What products are formed when the following...Ch. 6.11 - Prob. 31PCh. 6.11 - Prob. 32PCh. 6.11 - The following product was obtained from the...Ch. 6.12 - What characteristics must the reactant of a...Ch. 6.13 - Prob. 36PCh. 6.13 - What stereoisomers are obtained from each of the...Ch. 6.13 - Prob. 41PCh. 6.13 - Prob. 42PCh. 6.13 - Prob. 43PCh. 6.13 - Prob. 45PCh. 6.13 - Prob. 46PCh. 6.13 - Prob. 47PCh. 6.13 - Prob. 48PCh. 6.13 - Prob. 49PCh. 6.13 - Prob. 50PCh. 6.14 - Prob. 51PCh. 6.16 - Prob. 53PCh. 6.16 - Explain why 3-methykyclohexene should not be used...Ch. 6 - Prob. 55PCh. 6 - Prob. 56PCh. 6 - Prob. 57PCh. 6 - What is the major product of the reaction of...Ch. 6 - Give two names for each of the following:Ch. 6 - Prob. 60PCh. 6 - What are the products of the following reactions?...Ch. 6 - When 3-methyl-1-butene reacts with HBr, two alkyl...Ch. 6 - Draw curved arrows to show the flow of electrons...Ch. 6 - What reagents are needed to carry out the...Ch. 6 - Prob. 65PCh. 6 - Prob. 66PCh. 6 - Prob. 67PCh. 6 - What is more stable? a. CH3C+HCH3orCH3C+HCH2ClCh. 6 - Prob. 69PCh. 6 - a. Draw the product or products that will be...Ch. 6 - Prob. 71PCh. 6 - The second-order rate constant (in units of M1s1)...Ch. 6 - Which compound has the greater dipole moment?Ch. 6 - Prob. 74PCh. 6 - Prob. 75PCh. 6 - Prob. 76PCh. 6 - Prob. 77PCh. 6 - Prob. 78PCh. 6 - Prob. 79PCh. 6 - Prob. 80PCh. 6 - Prob. 81PCh. 6 - Prob. 82PCh. 6 - Prob. 83PCh. 6 - Prob. 84PCh. 6 - Prob. 85PCh. 6 - Prob. 86PCh. 6 - Draw the products of the following reactions. If...Ch. 6 - Prob. 88PCh. 6 - Prob. 89PCh. 6 - Prob. 90PCh. 6 - Two chemists at Dupont found that lCH2Znl is...Ch. 6 - Prob. 92PCh. 6 - Prob. 93PCh. 6 - What alkene gives the product shown after...Ch. 6 - Prob. 95PCh. 6 - Prob. 96PCh. 6 - Prob. 97PCh. 6 - Prob. 98PCh. 6 - Prob. 99PCh. 6 - Prob. 100PCh. 6 - Propose a mechanism for the following reaction:Ch. 6 - Prob. 102PCh. 6 - Prob. 103P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Consider the by-products that might be formed by E1 and/or E2 processes in the reaction of 2-methyl-2-butanol with HCl. Provide structures for these E1 and/or E2 products. Which alkene would be favored and why?arrow_forwardFor each reaction, give the expected solution product and predict whether the machanism will be first order (SN1) or second order (SN2) : a) 2-chloro-2-methylbutane +CH3COOH b) isobutyl bromide + NaOMe c) 1-iodo-1-methylcyclohexane + CH3CH2OHarrow_forwardQ3. 2-Bromopentane, when treated with alcoholic KOH yields a mixture of three alkenes A, B and C. Identify A, B and C. Which is predominant? Q4 Which statement below about Sn1 reactions is incorrect? (A) SN1 reactions are stepwise and have intermediates. (B) The slow step in a SN1 reaction is formation of the carbocation intermediate. (C) SN1 reactions have first order kinetics which means only the alkyl halide is involved in the rate limiting step. (D) The products of a SN1 reaction will be a pair of enantiomers. (E) An aprotic solvent is best for Sn1 reactions as they tend to help stabilize carbocation intermediates.arrow_forward
- What are the characteristics of the a good nucleophile for SN2? How does a chemist tell a strong versus weak or bulky versus not bulky nucleophile? What is the best type of solvent for this reaction? Please define the terms. Thank you.arrow_forwardsummarize in a table how SN2 type reactions are characterized in terms of the following criteria: (1) choice of nucleophile; (2) substrate (RX); (3) solvent; (4) kinetics (order of reaction and rate law); (5) stereochemistry; (6) possibility of rearrangements.arrow_forwardShow stereo chemistry and possible outcomes and the steps for the reaction.arrow_forward
- a) Write down the products that will occur when you extract HBr from 2-bromo-3-methyl butane in a basic medium, State the reaction conditions. Show which product is the main product. b) Does the main product show the geometric isomer, so please write together. If it shows write the isomers. c) Write the product that will be formed when the main product reacts with KMnO4 in a basic environment in coldarrow_forwardThe 3D image below is that of an allylic carbocation intermediate formed by the protonation of a conjugated diene with HBr. Draw structural formulas for the final reaction products.arrow_forwardExplain why E2 reactions are also called β-elimination reactions.arrow_forward
- In the chemical reaction between 1-Bromobutane and ethanolic silver nitrate solution, a precipitate is barely formed. Using the skeletal structures of the molecules, write out the skeletal structure of each reaction (don't include the mechanism). Identify what the nucleophile is in each type of reaction, especially the SN1 reaction. Explain the reactivity of each electrophile/substrate in terms of whether they are tertiary, secondary, primary, etc. for each reaction. Include the overall chemical reaction.arrow_forwardwhat structure show the transition state for the rate determining step in the sn1 reaction with this picture?arrow_forwardWhat would be the major product obtained from the reaction of Br2 with 1-butene if the reaction were carried out in the solvent dichloromethane? Draw the molecule.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License