
Concept explainers
The volume flow rate of air in the steam heating system.
Answer to Problem 73P
The volume flow rate of air in the steam heating system is
Explanation of Solution
Given:
The inlet pressure of air
The inlet temperature of air
The outlet temperature of air
The inlet pressure of steam
The inlet pressure of steam
The outlet pressure of steam
The outlet temperature of steam
Calculation:
Draw the schematic diagram of the steam heating system.
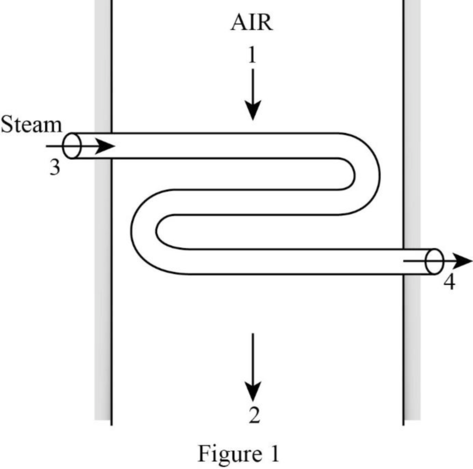
Write the expression for the mass balance.
Here, mass of the fluid entering into the system is
Write the energy rate balance equation for a control volume.
Here, total energy rate at inlet is
Write the ideal gas equation for specific volume of air
Here, gas constant for air is
Calculate the volume flow rate of the air at the inlet
Here, mass flow rate of air is
Refer Table A-1E, “Gas constant of common gases”, obtain the gas constant of air as
Refer Table A-2E, “Ideal – gas specific heats of common gases”, obtain the constant pressure specific heat of air as
Refer Table A-6E, “Superheated water”, obtain the inlet specific enthalpy
Refer Table A-4E of “Saturated water-Temperature table”, obtain the final specific enthalpy of saturated water at temperature
Rewrite Equation (I) for the mass flow rate of air
Here, mass flow rate of air at inlet is
Rewrite Equation (I) for the mass flow rate of steam
Here, mass flow rate of steam at inlet is
Rewrite Equation (II) for the energy balance in the steam heating system.
Substitute
Here, specific enthalpy of air at the inlet is
Substitute
Substitute
Substitute
Thus, the volume flow rate of the air in steam heating system is
Want to see more full solutions like this?
Chapter 6 Solutions
FUND.OF THRMAL FLD. SCI. W/ACC. CODE>C
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





