
Interpretation:
Structure and configuration of the products obtained in the given reactions has to be drawn.
Concept introduction:
Stereochemistry of addition reaction in
The dibromination or addition of bromin in alkenes by using
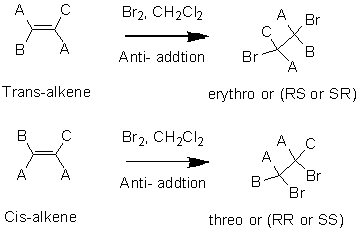
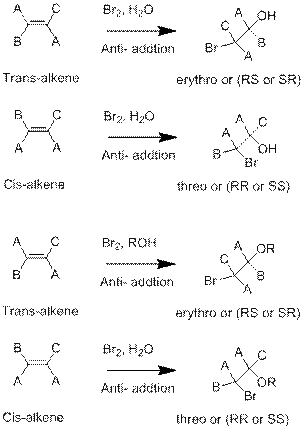
In the above reaction, the hydroxyl bromo derivative product will form when reagent
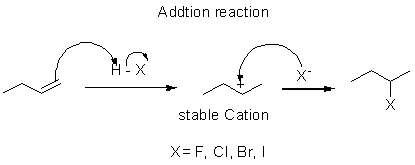
Hydrohalogenation:
The intermediate of addtion of hydrogen halide in alkene is carbocation so the stable carbocation is formed in the reaction then the helaide ion is added in it.
Markovnikov's rule:
It says the negative part of the reactant is added in the position of highly substituted carbon in alkene.
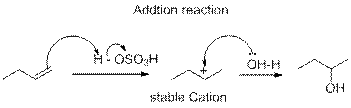
In the above reaction, the hydroxyl derivative will formed, when
Hydrogenation:
The reduction or addition of hydrogen in alkenes by using
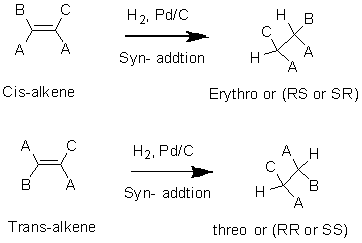
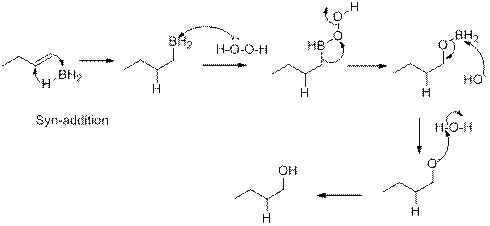
Hydroboration:
In the addition of
Want to see the full answer?
Check out a sample textbook solution
Chapter 6 Solutions
ORGANIC CHEMISTRY (LL)-W/MOD.MASTERING.
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT

