
Concept explainers
Interpretation:
Based on the structure given, the different types of stereoisomers for each of the conditions given are to be identified.
Concept introduction:
A chiral carbon atom is attached to four different atoms or groups of atoms. The number of stereoisomers is determined by using the formula
Stereoisomers arise from chiral centers as well as the arrangement of atoms or groups attached to the double bonded carbon atoms.
The pair of isomers designated
Enantiomers are non-superimposable mirror images of each other.
Stereoisomers that are not mirror images are diastereomers.
Answer to Problem 35P
Solution:
a) The number of stereoisomers represented by the given constitution is
b) If the substituents on the five-membered ring are cis to each other, then there are four stereoisomers represented by the given constitution.
c) If the butenyl side chain has the Z configuration of its double bond, the given constitutional isomer has four possible stereoisomers.
d) Stereochemically accurate representations of all the stereoisomers are as follows:
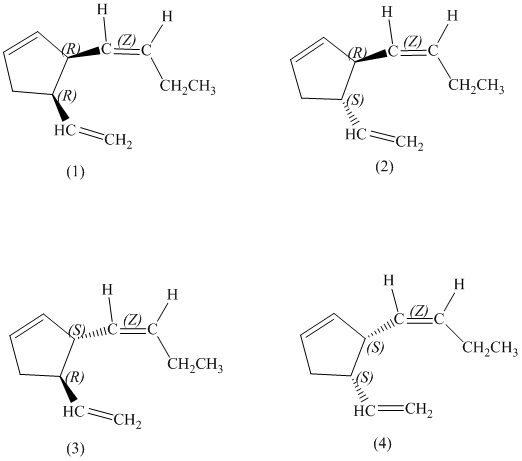
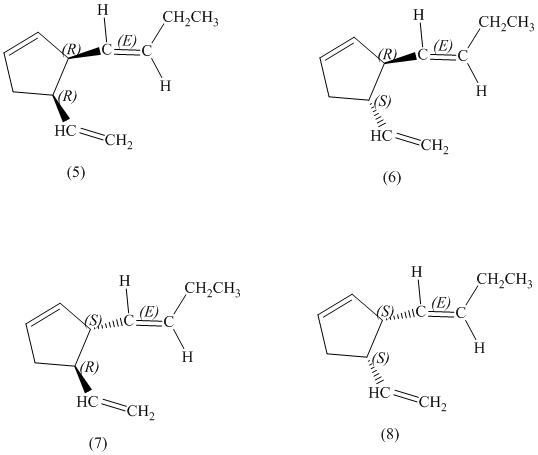
e) The pairs of enantiomers are as follows:
The pairs of diastereomers are as follows:
Explanation of Solution
a)
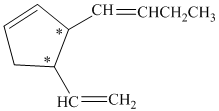
In the given structure, there are two chiral carbon atoms indicated by
Also, the double bond of butenyl side chain can form E and Z isomers, so the total number of possible stereoisomers will be:
Therefore, the number of stereoisomers represented by the given constitution is
b)
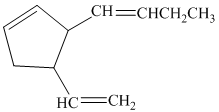
So, if the substituents on the five-membered ring are cis to each other, then the double bond in the butenyl side chain can be in the E or Z form. Thus, there are
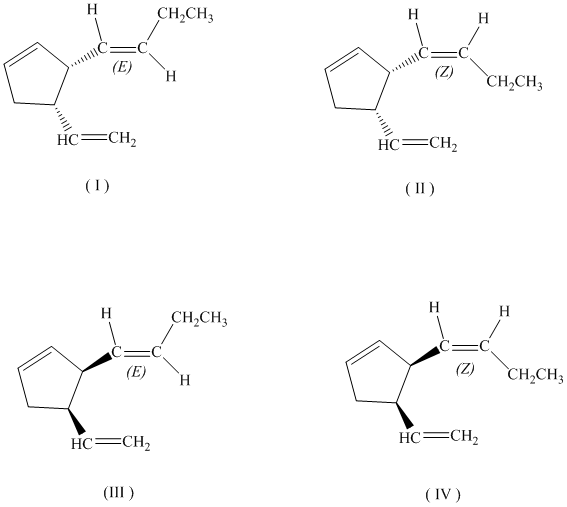
c)

If the butenyl side chain has the Z configuration of its double bond, the two substituents on the ring can be oriented in four different ways. The number of possible stereoisomers is
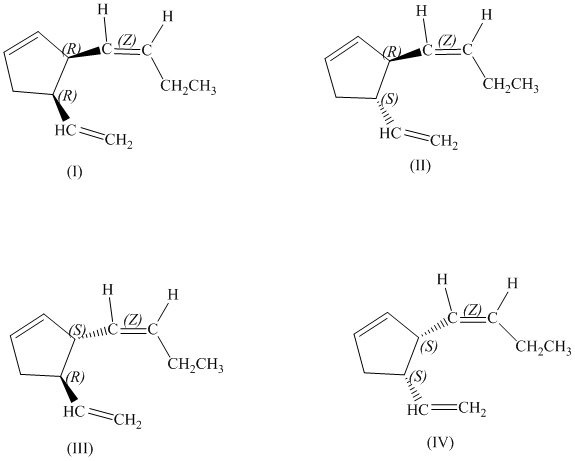
d)

There are total
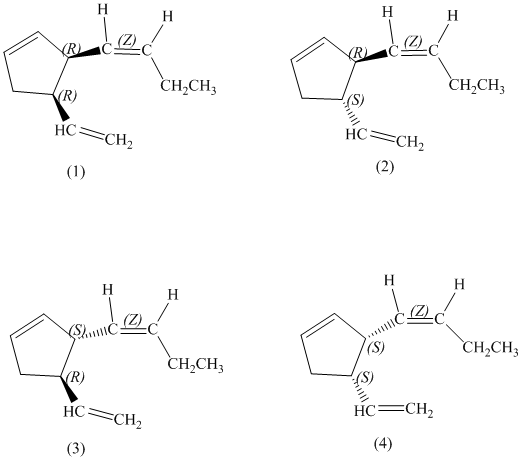
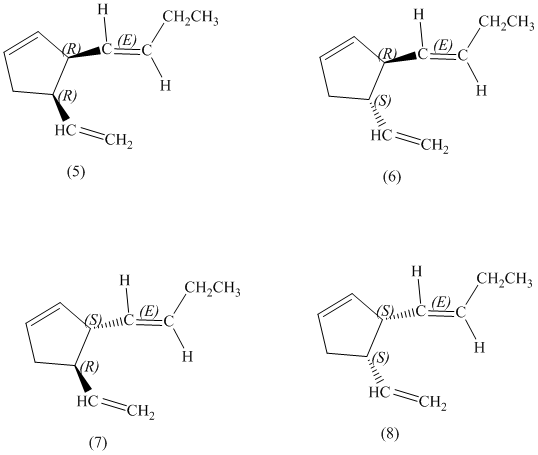
e)
Enantiomers are non-superimposable mirror images of each other. Diastereomers are the stereoisomers which are not mirror images.
The pairs of enantiomers are as follows:
The pairs of diastereomers are as follows:
Want to see more full solutions like this?
Chapter 7 Solutions
ORGANIC CHEMISTRY-W/STUD.SOLN.MAN.
- Ketones react with alcohols to yield products called acetals. Why does the all-cis isomer of 4-tert-butyl-1,3-cyclohexanediol react readily with acetone and an acid catalyst to form an acetal, but other stereoisomers do not react? In formulating your answer, draw the more stable chair conformations of all four stereoisomers and the product acetal for each one.arrow_forwardDraw the structure of the expected organic produect formed in the following reactions including correct relative stereochemistry; if the reaction is racemic indicate this by either drawing both enantiomers or drawing one and writing racemic. Assume all reagent are present in excess unless otherwise noted. If no reaction occurs, state "No Reaction".arrow_forwardWhich double bonds in the following natural products can exhibit stereoisomerism? Nerolidol is isolated from the angel's trumpet plant, caryophyllene is present in hemp, and humulene comes from hops.arrow_forward
- Draw the two major organic products of the reaction in ether. Show the stereochemistry of the products. Are the products single enantiomers or racemic mixtures?arrow_forwardThe absolute configuration of BAGO is (dextrorotatory, levorotatory, R, S). The melting point of its enantiomer will be (higher, lower, equal) than/with 197.2. The relative configuration of its enantiomer will be (dextrorotatory, levorotatory, R, S). The density of BAGO is (higher, lower, equal, twice) the density of its enantiomer.arrow_forwardWhat is the absolute stereochemistry at the purple carbons?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


