
Concept explainers
Draw Lewis structures and give the other information requested for the following molecules: (a) BF3, Shape: planar or nonplanar? (b) ClO3− Shape: planar or nonplanar? (c) HCN. Polar or nonpolar? (d) OF2. Polar or nonpolar? (c) NO2. Estimate the ONO bond angle.
a)
Interpretation: The Lewis structures of the given molecules should be drawn. To identify whether
Concept Introduction:
- Molecular geometry is the spatial arrangement of atoms in a molecule. It is the three dimensional arrangement of bonded atoms. Many chemical, physical and even biological properties depend on molecular geometry.
- Bond angle measured that made between two nearby bonds. The angles between two adjacent bonds are known as bond angle.
- Using VSEPR theory and Lewis structure, the exact geometry of a molecule can be obtained.
- In VSEPR, the geometry of the molecule is explained based on minimizing electrostatic repulsion between the molecules’ valence electrons around a central atom
- Lewis structures is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electronsthat may exist in the molecule.
- While formation of a bond, there is a chance that electronegativity between the atoms are high. It tends to make the bond partially ionic and are called polar molecule. When the electronegativity between two atoms is similar, sharing of electron in the bond is equal and is called nonpolar molecules.
Answer to Problem 7.105QP
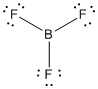 is the Lewis structure of
is the Lewis structure of
Explanation of Solution
Lewis structure of

Here boron is taken as the central atom with three terminal fluorine atoms since electronegativity of boron is less than fluorine. The total number of valence electrons is found to be 24, where each of the fluorine atoms contributes 6 electrons whereas boron contributes 3 electrons.
The 18 electrons getting after reducing two electrons for each bond from the total valence electron are distributed on terminal fluorine atom to complete the octet. After the distribution of electrons on the terminal atoms, each fluorine atoms gets 3 pairs of electrons.
Here the central atom boron atom does not have any lone pair of electrons whereas the terminal fluorine atoms have 3 pairs of electron. It is a
b)
Interpretation: The Lewis structures of the given molecules should be drawn. To identify whether
Concept Introduction:
- Molecular geometry is the spatial arrangement of atoms in a molecule. It is the three dimensional arrangement of bonded atoms. Many chemical, physical and even biological properties depend on molecular geometry.
- Bond angle measured that made between two nearby bonds. The angles between two adjacent bonds are known as bond angle.
- Using VSEPR theory and Lewis structure, the exact geometry of a molecule can be obtained.
- In VSEPR, the geometry of the molecule is explained based on minimizing electrostatic repulsion between the molecules’ valence electrons around a central atom
- Lewis structures is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electronsthat may exist in the molecule.
- While formation of a bond, there is a chance that electronegativity between the atoms are high. It tends to make the bond partially ionic and are called polar molecule. When the electronegativity between two atoms is similar, sharing of electron in the bond is equal and is called nonpolar molecules.
Answer to Problem 7.105QP
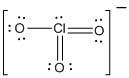 is the Lewis structure of
is the Lewis structure of
Explanation of Solution
Lewis structure of

Here chlorine is taken as the central atom with three terminal oxygen atoms. The total number of valence electrons is found to be 25 where each of the oxygen atoms contributes 6 electrons whereas chlorine contributes 5 electrons. The charge of the whole molecule is -1 making the total number of valence electrons 26.
The 20 electrons getting after reducing two electrons for each bond from the total valence electron are distributed on terminal oxygen atom to complete the octet. Remaining 2 electrons were distributed on central phosphorous atom. Since the atoms do not obey the octet rule two double bonds are made between chlorine and oxygen atoms.
In the case of
c)
Interpretation: The Lewis structures of the given molecules should be drawn. To identify whether
Concept Introduction:
- Molecular geometry is the spatial arrangement of atoms in a molecule. It is the three dimensional arrangement of bonded atoms. Many chemical, physical and even biological properties depend on molecular geometry.
- Bond angle measured that made between two nearby bonds. The angles between two adjacent bonds are known as bond angle.
- Using VSEPR theory and Lewis structure, the exact geometry of a molecule can be obtained.
- In VSEPR, the geometry of the molecule is explained based on minimizing electrostatic repulsion between the molecules’ valence electrons around a central atom
- Lewis structures is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electronsthat may exist in the molecule.
- While formation of a bond, there is a chance that electronegativity between the atoms are high. It tends to make the bond partially ionic and are called polar molecule. When the electronegativity between two atoms is similar, sharing of electron in the bond is equal and is called nonpolar molecules.
Answer to Problem 7.105QP
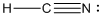 is the Lewis structure of
is the Lewis structure of
Explanation of Solution
Lewis structure of
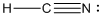
Here carbon is taken as the central atom with onenitrogen and one hydrogen atom in the terminal position since electronegativity of carbon is less. The total number of valence electrons is found to be 10, where each of the nitrogen atoms contributes 5 electrons whereas carbon contributes 4 electrons whereas hydrogen atom contributes only one electron.
The 6 electrons getting after reducing two electrons for each bond from the total valence electron are distributed on terminal nitrogen atom to complete the octet. Since the atoms do not obey the octet rule a triple bond is made between carbon and nitrogen atom.
In
d)
Interpretation: The Lewis structures of the given molecules should be drawn. To identify whether
Concept Introduction:
- Molecular geometry is the spatial arrangement of atoms in a molecule. It is the three dimensional arrangement of bonded atoms. Many chemical, physical and even biological properties depend on molecular geometry.
- Bond angle measured that made between two nearby bonds. The angles between two adjacent bonds are known as bond angle.
- Using VSEPR theory and Lewis structure, the exact geometry of a molecule can be obtained.
- In VSEPR, the geometry of the molecule is explained based on minimizing electrostatic repulsion between the molecules’ valence electrons around a central atom
- Lewis structures is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electronsthat may exist in the molecule.
- While formation of a bond, there is a chance that electronegativity between the atoms are high. It tends to make the bond partially ionic and are called polar molecule. When the electronegativity between two atoms is similar, sharing of electron in the bond is equal and is called nonpolar molecules.
Answer to Problem 7.105QP
Answer
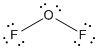 is the Lewis structure of
is the Lewis structure of
Explanation of Solution
Lewis structure of

Here oxygenis taken as the central atom with two terminal fluorine atoms since electronegativity of oxygen is less than fluorine. The total number of valence electrons is found to be 20, where each of the fluorine atoms contributes 7 electrons whereas oxygen contributes 6 electrons.
The 16 electrons getting after reducing two electrons for each bond from the total valence electron are distributed on silicon atom to complete the octet. After the distribution of electrons on the terminal atoms, each fluorine atoms gets 3 pairs of electrons. The remaining 4 electrons are distributed to the oxygen atom.
The central atom has two lone pairs thus it has four electron domains so it have bent geometry. Due to the difference in the electronegativity the bonds are polar. But due to the presence of lone pair on the central atom, the molecule is not symmetrical. The dipole vectors do not cancel each other thus having a total dipole moment on the molecule. So it is a polar molecule.
e)
Interpretation: The Lewis structures of the given molecules should be drawn. To identify whether
Concept Introduction:
- Molecular geometry is the spatial arrangement of atoms in a molecule. It is the three dimensional arrangement of bonded atoms. Many chemical, physical and even biological properties depend on molecular geometry.
- Bond angle measured that made between two nearby bonds. The angles between two adjacent bonds are known as bond angle.
- Using VSEPR theory and Lewis structure, the exact geometry of a molecule can be obtained.
- In VSEPR, the geometry of the molecule is explained based on minimizing electrostatic repulsion between the molecules’ valence electrons around a central atom
- Lewis structures is also known as Lewis dot structures which represents the bonding between atoms of a molecule and the lone pairs of electronsthat may exist in the molecule.
- While formation of a bond, there is a chance that electronegativity between the atoms are high. It tends to make the bond partially ionic and are called polar molecule. When the electronegativity between two atoms is similar, sharing of electron in the bond is equal and is called nonpolar molecules.
Answer to Problem 7.105QP
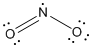 is the Lewis structure of
is the Lewis structure of
Explanation of Solution
Lewis structure of

Here nitrogen is taken as the central atom with two terminal oxygen atoms since electronegativity of oxygen is less than fluorine. The total number of valence electrons is found to be 20, where each of the fluorine atoms contributes 7 electrons whereas oxygen contributes 6 electrons.
The 12 electrons getting after reducing two electrons for each bond from the total valence electron are distributed on terminal oxygen atom to complete the octet. The remaining one electron is distributed to the nitrogen atom. Since the atoms do not obey the octet rule a double bond is made between one of the oxygen and nitrogen atom.
Want to see more full solutions like this?
Chapter 7 Solutions
Chemistry: Atoms First
- In each of the following molecules, a central atom is surrounded by a total of three atoms or unshared electron pairs: SnCl2, BCl3, SO2. In which of these molecules would you expect the bond angle to be less than 120? Explain your reasoning.arrow_forwardExplain in terms of bonding theory why all four hydrogen atoms of allene, H2CCCH2, cannot lie in the same plane.arrow_forwardPredict die molecular structure and bond angles for each molecule or ion in Exercises 88 and 94. a. POCl3, SO42, XeO4, PO43, ClO4 b. NF3, SO32, PO33, ClO3 c.ClO2, SCl2, PCl2 d. Considering your answers to parts a, b, and c. what conclusions can you draw concerning the structures of species containing the same number of atoms and the same number of valence electrons? (O3), sulfur dioxide, and sulfur trioxide.arrow_forward
- It is possible to write a simple Lewis structure for the SO42- ion, involving only single bonds, which follows the octet rule. However, Linus Pauling and others have suggested an alternative structure, involving double bonds, in which the sulfur atom is surrounded by six electron pairs. (a) Draw the two Lewis structures. (b) What geometries are predicted for the two structures? (c) What is the hybridization of sulfur in each case? (d) What are the formal charges of the atoms in the two structures?arrow_forwardDraw the Lewis Structure of carbonate ion, CO32-. Please include the resonance structures and the formal charges on each atom. Consider the compound: sulfur tetrafluoride. a. Draw the Lewis Structure, b. What is its AXE notation? c. What is its Molecular geometry? d. What is its Electron pair geometry? e. Is it polar or non-polar? 3. Consider the Ne2 molecule. a. Draw its molecular orbital diagram, b. Compute the bond order, c. Is it more stable compared to its non-bonded form?arrow_forward3. How do the P-Ci single bond lengths in PCl5, PCl4+, and PCl6- generally compare? 4. Identify the type of bonding the central atom undergoes in PCl5 and PCl6-.arrow_forward
- Methyl isocyanate, CH3NCO, was made infamous in 1984when an accidental leakage of this compound from a storagetank in Bhopal, India, resulted in the deaths of about3800 people and severe and lasting injury to many thousandsmore. (a) Draw a Lewis structure for methyl isocyanate.(b) Draw a ball-and-stick model of the structure,including estimates of all the bond angles in the compound.(c) Predict all the bond distances in the molecule.(d) Do you predict that the molecule will have a dipolemoment? Explain.arrow_forwardBetween Cl2O and XeF4 which one has dipole moment and which one is not?arrow_forwardExcept for nitrogen, the elements of Group 5A(15) all form pentafluorides, and most form pentachlorides. The chlorine atoms of PCl₅ can be replaced with fluorine atoms one at a timeto give, successively, PCl₄F, PCl₃F₂, ... , PF₅. (a) Given the sizesof F and Cl, would you expect the first two F substitutions to beat axial or equatorial positions? Explain. (b) Which of the five fluorine-containing molecules have no dipole moment?arrow_forward
- 9.14 (a) Methane 1CH42 and the perchlorate ion 1ClO4- 2 are bothdescribed as tetrahedral. What does this indicate about theirbond angles? (b) The NH3 molecule is trigonal pyramidal,while BF3 is trigonal planar. Which of these molecules is flat?arrow_forwardComplete the following resonance structures for POCl3. a. Would you predict the same molecular structure from each resonance structure? b. What is the hybridization of P in each structure? c. What orbitals can the P atom use to form the π bond in structure B? d. Which resonance structure would be favoured on the basis of formal charges?arrow_forwardWhich species has the smaller bond angle, H3O + or H2O? Explain?arrow_forward
 Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning





