
Concept explainers
(a)
Interpretation:
Curved arrow and the product formed for the given nucleophilic addition step are to be drawn.
Concept introduction:
The curved arrow drawn from the nucleophile (electron rich species) to the polar pi-bond (electron poor species) represents the flow of electrons from an electron rich site to an electron poor site. The second curved arrow is drawn from the center of double or triple bond to the electronegative atom. A new bond is formed between the nucleophile and the electron deficient atom.
Answer to Problem 7.26P
The curved arrow and the product formed in the given nucleophilic addition step is drawn as:
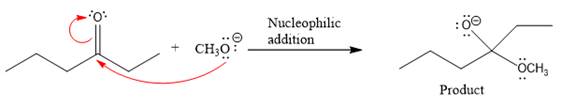
Explanation of Solution
In the given nucleophilic addition step is:
In this step,
Thus, the curved arrows for the given nucleophilic addition step are drawn as:
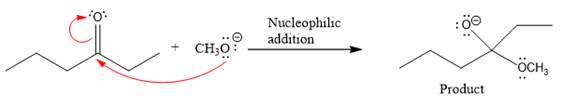
The new bond in the product is formed between the electron rich O atom from the nucleophile and the electron poor C atom of the carbonyl group.
The curved arrow represents the flow of electrons from an electron rich site to an electron poor site.
(b)
Interpretation:
Curved arrow and the product formed for the given nucleophilic addition step are to be drawn.
Concept introduction:
The curved arrow drawn from the nucleophile (electron rich species) to the polar pi-bond (electron poor species) represents the flow of electrons from an electron rich site to an electron poor site. The second curved arrow is drawn from the center of double or triple bond to the electronegative atom. A new bond is formed between the nucleophile and the electron deficient atom.
Answer to Problem 7.26P
The curved arrow and the product formed in the given nucleophilic addition step is drawn as:
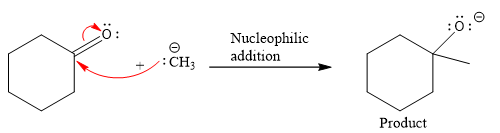
Explanation of Solution
In the given nucleophilic addition step is:
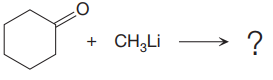
In this step
Thus, the curved arrows for the given nucleophilic addition step are drawn as:

The new bond in the product is formed between electron rich C atom from the nucleophile and electron poor C atom of the carbonyl group.
The curved arrow represents the flow of electrons from electron rich site to electron poor site.
(c)
Interpretation:
Curved arrow and the product formed for the given nucleophilic addition step are to be drawn.
Concept introduction:
The curved arrow drawn from the nucleophile (electron rich species) to the polar pi-bond (electron poor species) represents the flow of electron from an electron rich site to an electron poor site. The second curved arrow is drawn from the center of double or triple bond to the electronegative atom. A new bond is formed between the nucleophile and the electron deficient atom.
Answer to Problem 7.26P
The curved arrow and the product formed in the given nucleophilic addition step is drawn as:
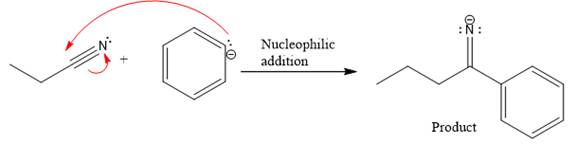
Explanation of Solution
In the given nucleophilic addition step is:
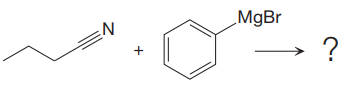
In this step
Thus, the curved arrows for the given nucleophilic addition step are drawn as:

The new bond in the product is formed between electron rich C atom from the nucleophile and electron poor C atom.
Curved arrow represents the flow of electrons from electron rich site to electron poor site.
(d)
Interpretation:
Curved arrow and the product formed for given nucleophilic addition step are to be drawn.
Concept introduction:
The curved arrow drawn from the nucleophile (electron rich species) to the polar pi-bond (electron poor species) represents the flow of electron from an electron rich site to an electron poor site. The second curved arrow is drawn from the centre of double or triple bond to the electronegative atom. A new bond is formed between the nucleophile and the electron deficient atom.
Answer to Problem 7.26P
The curved arrow and the product formed in the given nucleophilic addition step is drawn as:
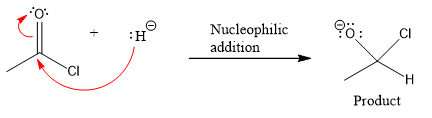
Explanation of Solution
In the given nucleophilic addition step is:
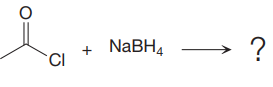
In this step
Thus, the curved arrows for the given nucleophilic addition step are drawn as:

The new bond in the product is formed between the electron rich H atom from the nucleophile and the electron poor C atom of the carbonyl group.
Curved arrow represents flow of electrons from electron rich site to electron poor site.
(e)
Interpretation:
Curved arrow and the product formed for given nucleophilic addition step are to be drawn.
Concept introduction:
The curved arrow drawn from the nucleophile (electron rich species) to the polar pi-bond (electron poor species) represents the flow of electron from an electron rich site to an electron poor site. The second curved arrow is drawn from the center of double or triple bond to the electronegative atom. A new bond is formed between the nucleophile and the electron deficient atom.
Answer to Problem 7.26P
The curved arrow and the product formed in the given nucleophilic addition step is drawn as:
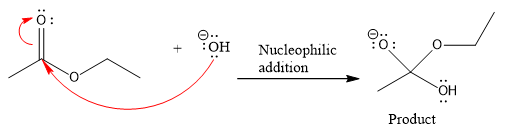
Explanation of Solution
In the given nucleophilic addition step is:
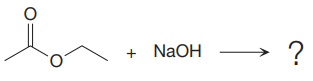
In this step
Thus, the curved arrows for the given nucleophilic addition step are drawn as:

The new bond in the product is formed between electron rich O atom from the nucleophile and electron poor C atom of the carbonyl group.
Curved arrow represents the flow of electrons from electron rich site to electron poor site.
(f)
Interpretation:
Curved arrow and the product formed for the given nucleophilic addition step are to be drawn.
Concept introduction:
The curved arrow drawn from the nucleophile (electron rich species) to the polar pi-bond (electron poor species) represents the flow of electron from an electron rich site to an electron poor site. The second curved arrow is drawn from the center of double or triple bond to the electronegative atom. A new bond is formed between the nucleophile and the electron deficient atom.
Answer to Problem 7.26P
The curved arrow and the product formed in the given nucleophilic addition step is drawn as:

Explanation of Solution
In the given nucleophilic addition step is:
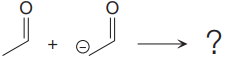
In the above step,
Thus, the curved arrows for the given nucleophilic addition step are drawn as:

The new bond in the product is formed between the electron rich C atom from the nucleophile and the electron poor C atom of the carbonyl group.
Curved arrow represents the flow of electrons from electron rich site to electron poor site.
Want to see more full solutions like this?
Chapter 7 Solutions
ORGANIC CHEMISTRY PRINCIPLES & MECHANISM
- Be sure to include all lone pair electrons and nonzero formal charges. Step 1 Step 2 i Draw H3CO¯, and then add curved arrow notation showing nucleophilic addition.arrow_forwardCircle the reactant that is a nucleophile and draw the mechanism using the appropriate arrows for the following reaction. Dalarrow_forwardDraw the products of attached reaction by following the curved arrows.arrow_forward
- Follow the curved arrows and draw the product of this reaction. Ph • You do not have to consider stereochemistry.arrow_forwardCircle the sites on diagram please. 7. Identify the nucleophilic and electrophilic sites in the reactants of the following reaction. Li-Mearrow_forwardPlease correct answer and don't use hend raitingarrow_forward
- CO2 Follow the curved arrows and draw the product of this reaction. You do not have to consider stereochemistry.arrow_forwardHow can we determine whether the equilibrium will favor products in a nucleophilic substitution?arrow_forwardDraw the complete mechanism of each pair of reactants including any favorable rearrangements and all important resonance structures of all intermediates. a. Which reaction has a lower PE carbocation intermediate? b. Draw an energy diagram showing the reaction profiles of both reactions in the previous question. Use a dotted line for the first pair of reactants and a solid line for the second pair of reactants. (Assume the energy of the starting materials and products are the same for both pairs and the reactions are neither uphill nor downhill on net. c. Mark points on the energy diagram corresponding to each carbocation in your mechanisms.arrow_forward
- Please draw arrow-pushing reaction mechanism and identify number of electrons involved here. This is an electrolytic reactionarrow_forwardBonus Question: If you deemed the previous reaction to be unsuccessfull, propose a reaction or synthesis that would successfully produce the desired ether product (shown again to the side). You may use any reaction you know of.arrow_forwardAdd the curved arrow notation to this proton transfer reaction. Then classify each starting material according to its reactivity role in the reaction. ++ -Ö-Harrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
