
Concept explainers
The standard procedure for synthesizing a compound is the stepwise progress toward a target molecule by forming individual bonds through single reactions. Typically, the product of each reaction is isolated and purified before the next reaction in the sequence is carried out. One of the ways nature avoids this tedious practice of isolation and purification is by the use of a domino sequence in which each new product is built on a preexisting one in stepwise fashion. A great example of a laboratory domino reaction is William S. Johnson’s elegant synthesis of the female hormone progesterone. Johnson first constructed the polyunsaturated monocyclic 3° alcohol (A) and then, in an acid-induced domino reaction, formed compound B, which he then converted to progesterone.
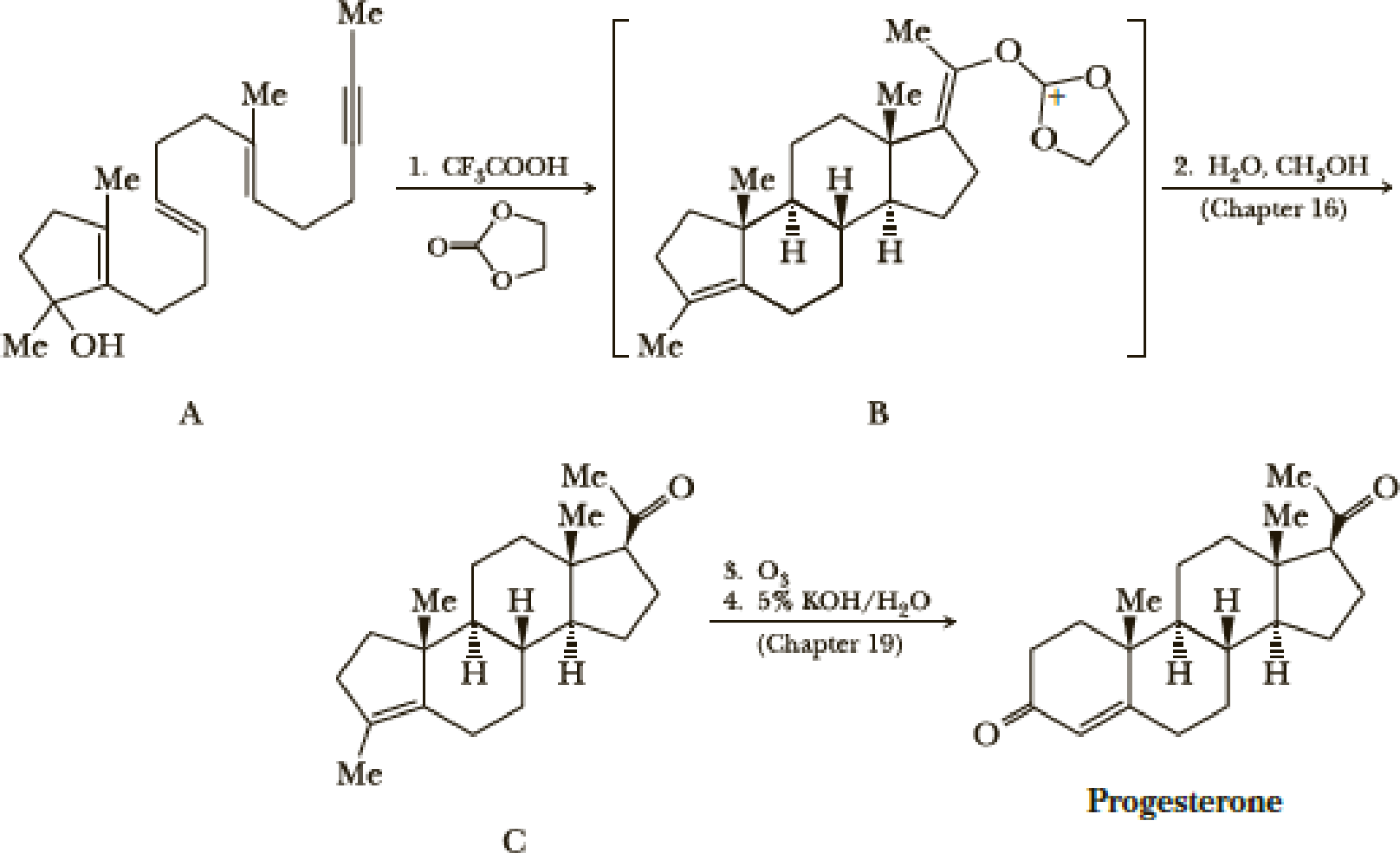
A remarkable feature of this synthesis is that compound A, which has only one stereo-center, gives compound B, which has five stereocenters, each with the same configuration as those in progesterone. We will return to the chemistry of Step 2 in Section 16.7 and to the chemistry of Steps 3 and 4 in Chapter 19. In this problem, we focus on Step 1.
- (a) Assume that the domino reaction in Step 1 is initiated by protonation of the 3° alcohol in compound A followed by loss of H2O to give a 3° carbocation. Show how the series of reactions initiated by the formation of this cation gives compound B.
- (b) If you have access to a large enough set of molecular models or to a computer modeling program, build a model of progesterone and describe the conformation of each ring. There are two methyl groups and three hydrogen atoms at the set of ring junctions in progesterone. Which of these five groups occupies an equatorial position? Which occupies an axial position?
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry, Loose-leaf Version
- 10. Terpene is a class of naturally occurring molecules, found in many essential oils from plants, that share the same alkene features. All terpenes have a similar 5 carbon unit that includes an alkene thought to be biologically synthesized from the molecule isoprene. All terpenes are biosynthesized from molecules with the good leaving group: pyrophosphate (OPP). Loss of this OPP leads to the formation of a resonance stabilized carbocation. The new carbocation can undergo a nucleophilic attack from a T-bond as seen in acid-catalyzed hydration and hydrohalogenation, forming a new carbocation. This new carbocation can undergo an E1 reaction to form a new alkene. Add the curved arrows of the following reaction. hopp COPP D 5 R-carvone (spearmint oil) - Q Search 40 6 & COPP 1 COPP COPParrow_forwardThe SN2 type reaction will end up with the substitution 2903 the at the reaction center of the reaction. 903 182903 SEY 9037arrow_forwardFunctional groups such as alkynes react the same in complex molecules as they do in simpler structures. The following example of alkyne reaction were taken from syntheses carried out in the research group of E. J. Corey at Harvard University. You can assume that the reactions listed involve only the alkyne, not any of the functional groups present in the molecules. Draw the expected products for the following reaction .arrow_forward
- H3C CH3 H3C NA C→XT Br Br₂ CH₂Cl₂ H3C Electrophilic addition of bromine, Br₂, to alkenes yields a 1,2-dibromoalkane. The reaction proceeds through a cyclic intermediate known as a bromonium ion. The reaction occurs in an anhydrous solvent such as CH₂Cl₂. CH3 Br In the second step of the reaction, bromide is the nucleophile and attacks at one of the carbons of the bromonium ion to yield the product. Due to steric clashes, the bromide ion always attacks the carbon from the opposite face of the bromonium ion so that a product with anti stereochemistry is formed. Draw curved arrows to show the movement of electrons in this step of the mechanism. Arrow-pushing Instructions Br CH3 H3C CH3arrow_forwardAlcohols are important for organic synthesis, especially in situations involving alkenes. The alcohol might be the desired product, or the OH group might be transformed into another functional group via halogenation, oxidation, or perhaps conversion to a sulfonic ester derivative. Formation of an alcohol from an alkene is particularly powerful because conditions can be chosen to produce either the Markovnikov or non-Markovnikov product from an unsymmetrical alkene. Using your reaction roadmap as a guide, show how to convert 4-methyl-1-pentene into 5-methylhexanenitrile. You must use 4-methyl-1-pentene and sodium cyanide as the source of all carbon atoms in the target molecule. Show all reagents needed and all molecules synthesized along the way.arrow_forwardCompounds containing a phenol group may work as ANTIOXIDANTS to prevent free radical damage. This is accomplished when a free radical (or UV light) encounters a phenol group, turning the phenol group into a radical. However, contrary to typical radical behavior, the structure of the phenol radical can neutralize (or quench) the unpaired electron. Specifically, the phenol structure neutralizes (or quenches) the unpaired radical electron by doing the following: taking the electron and The correct name (or abbreviation) of an example compound (discussed in the lecture videos) containing a phenol group with antioxidant properties is:arrow_forward
- Consider this reaction: Br CH;OH Br-Br H3CO The mechanism proceeds through a first cationic intermediate, intermediate 1. Nucleophilic attack leads to intermediate 2, which goes on to form the final product. In cases that involve a negatively charged nucleophile, the attack of the nucleophile leads directly to the product. Br + CH3OH Br Intermediate 1 Intermediate 2 (product) In a similar fashion, draw intermediate 1 and intermediate 2 (final product) for the following reaction. OH + Br2 + HBr Br racemic mixturearrow_forwardConsider the given reaction in which NC−NC− is the nucleophile and CH3CNCH3CN is the solvent. The reactant molecule has a structure with solid and dashed wedge bonds. A solid wedge () is used to show the bond that is above the plane of the paper, and a dashed wedge () is used to show the bond that is behind the plane of the paper. Draw the product of the following reaction:arrow_forwardWhich of the following correctly completes the reaction? AICI3 → ? 2AICI3 → 2Al + 3CI2 O AICI3 → AICI + Cl2 AICI3 → Al + 3CI AICI3 → Al + Cl3arrow_forward
- 7. The reaction of methoxide anion with bromoethane to yield the ether ethyl methyl ether and the bromide anon (Br-) is an excellent example of a general reaction type called Sy2 (substitution nucleophilic bimolecular): CH,0+ CH,СH-Br a CH3-0-CH,СH; + Br- a. Change in enthalpy is -103 kJ/mol; the change in entropy is + 0.025 kJ/mol-K. Calculate DG at 300K. b. Is the reaction endergonic or exergonic? c. Is the reaction endothermic or exothermic? d. Use curved arrows to show the complete mechanism. Reaction of 2-methyl-1-butene with H-Cl could yield TWO alkyl chloride products. Draw and name 8. them.arrow_forwardAlcohols are acidic in nature. Therefore, a strong base can abstract the acidic hydrogen atom of the alcohol in a process known as deprotonation. The alcohol forms an alkoxide ion by losing the proton attached to the oxygen atom of the hydroxyl ( -OH) group. The alkoxide formed can act as a base or a nucleophile depending on the substrate and reaction conditions. However, not all bases can abstract the acidic proton of alcohols and not all alcohols easily lose the proton. Deprotonation depends on the strength of the base and the acidity of the alcohol. Strong bases, such as NaNH2, can easily abstract a proton from almost all alcohols. Likewise, more acidic alcohols lose a proton more easily. Determine which of the following reactions would undergo deprotonation based on the strength of the base and the acidity of the alcohol. Check all that apply. ► View Available Hint(s) CH3CH,OH + NH3 →CH,CH,O-NH CH3 CH3 H3C-C-H+NH3 → H3 C-C-H OH O-NH CH3CH2OH + NaNH, → CH3CH,O-Na* + NH3 CHC12 Cl₂…arrow_forwardPredict the product of the following organic reaction: CH₂ CH O || C–CH2–CH=CH—(CH2)3 CH3 (CH₂)3 CH=CH- - CH₂ CH3 + 4 H₂ Ni CH,−O -C–CH2–CH=CH–CH=CH–CH2–CH3 Specifically, in the drawing area below, draw the chemical structure of the product P. If there is no product, because this reaction won't happen, check the No reaction box under the drawing area. Click and drag to start drawing a structure. × Ś +arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
