
Concept explainers
(a)
Interpretation:
The Lewis formula of
Concept Introduction:
Lewis Structure: A Lewis structure shows a covalent bond as pair of electrons shared between two atoms.
Procedure to write Lewis formulas:
- The symbols of the atoms that are bonded together in the molecule next to one another are arranged.
- The total number of valence electrons in the molecule is calculated by adding the number of valence electrons for all the atoms in the molecules. If the species is an ion, then the charge of ion into account by adding electrons, if it is a negative ion or subtracting electrons if it is a positive ion.
- A two-electron covalent bond is represented by placing a line between the atoms, which are assumed to be bonded to each other.
- The remaining valence electrons as lone pairs about each atom are arranged so that the octet rule is satisfied for each other.
Formal charge (F.C): The charges that assigned to each atom in a molecule or ion by a set of arbitrary rules and don not actually represent the actual charges on the atoms are called as formal charges.
The formal charge is calculated using the formula,
The Lewis structure with zero formal charge or least separated formal charges is the preferred structure of the molecule.
(a)
Explanation of Solution
The total number of valence electrons in
Number of valence electrons in oxygen=
Number of valence electrons in chlorine=
Since there is a negative charge present in ion, one electron is added.
The total number of valence electrons is thirty-two.
One chlorine atom forms four bonds with oxygen that is eight electrons are used to form bonds and the remaining twenty-four atoms are used to satisfy the octet rule of oxygen atoms.
The Lewis formula is,
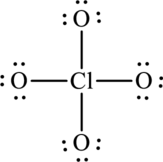
The formal charge for each atom is calculated as,
Formal charge on chlorine=
Formula charge on oxygen=
The Lewis formula is,
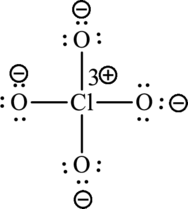
A large charge separation can be seen in the structure, this can be avoided by adding three pi bonds between oxygen and chlorine. The chlorine atom can expand the octet rule since it belongs to period three. The final Lewis structure is,
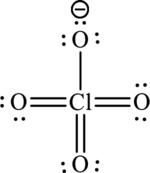
(b)
Interpretation:
The Lewis formula of
Concept Introduction:
Refer to part (a).
(b)
Explanation of Solution
The total number of valence electrons in
Number of valence electrons in oxygen=
Number of valence electrons in chlorine=
The total number of valence electrons is thirteen.
One chlorine atom forms a bond with oxygen atom that is two electrons are used to form bonds and the remaining nine electrons are used to satisfy the octet rule of oxygen and chlorine atoms.
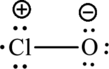
The Lewis formula is,
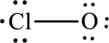
The formal charge for each atom is calculated as,
Formal charge on chlorine=
Formula charge on oxygen=
The Lewis formula is,
(c)
Interpretation:
The Lewis formula of
Concept Introduction:
Refer to part (a).
(c)
Explanation of Solution
The total number of valence electrons in
Number of valence electrons in oxygen=
Number of valence electrons in chlorine=
Since there is a negative charge present in ion, one electron is added.
The total number of valence electrons is twenty-six.
One chlorine atom forms three bonds with oxygen that is six electrons are used to form bonds and the remaining twenty atoms are used to satisfy the octet rule of oxygen atoms.
The Lewis formula is,
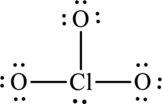
The formal charge for each atom is calculated as,
Formal charge on chlorine=
Formula charge on oxygen=
The Lewis formula is,
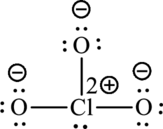
A large charge separation can be seen in the structure, this can be avoided by adding two pi bonds between oxygen and chlorine. The chlorine atom can expand the octet rule since it belongs to period three. The final Lewis structure is,
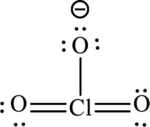
(d)
Interpretation:
The Lewis formula of
Concept Introduction:
Refer to part (a).
(d)
Explanation of Solution
The total number of valence electrons in
Number of valence electrons in oxygen=
Number of valence electrons in chlorine=
The total number of valence electrons is nineteen.
One chlorine atom forms two bonds with oxygen atom that is four electrons are used to form bonds and the remaining fifteen atoms are used to satisfy the octet rule of oxygen atoms.
The Lewis formula is,
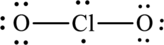
The formal charge for each atom is calculated as,
Formal charge on chlorine=
Formula charge on oxygen=
The Lewis formula becomes,
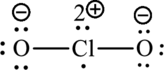
A large charge separation can be removed, by adding two pi bonds between chlorine and oxygen. Since chlorine belongs to third period, it can expand the octet rule. The final Lewis structure is,
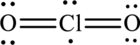
(e)
Interpretation:
The Lewis formula of
Concept Introduction:
Refer to part (a).
(e)
Explanation of Solution
The total number of valence electrons in
Number of valence electrons in oxygen=
Number of valence electrons in chlorine=
Since there is a negative charge present, one electron is added.
The total number of valence electrons is fourteen.
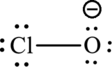
One chlorine atom forms a bond with oxygen atom that is two electrons are used to form bonds and the remaining twelve atoms are used to satisfy the octet rule of oxygen and chlorine atoms.
The Lewis formula is,
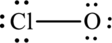
The formal charge for each atom is calculated as,
Formal charge on chlorine=
Formula charge on oxygen=
The Lewis formula is,
Want to see more full solutions like this?
Chapter 7 Solutions
GENERAL CHEMISTRY ACHIEVE ACCESS W/BOOK
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





