
Concept explainers
Muscalure, the sex pheromone of the common housefly, can be prepared by a reaction sequence that uses two nucleophilic substitutions. Identify compounds A-D in the following synthesis of muscalure.
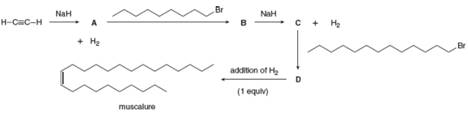
Interpretation: Compounds A, B, C and D are to be identified in the given synthesis of muscalure.
Concept introduction: The replacement or substitution of one functional group with another different functional group in any chemical reaction is termed as substitution reaction. The electron rich chemical species that contains negative charge or lone pair of electrons are known as a nucleophile. In a nucleophilic substitution reaction, nucleophile takes the position of leaving group by attacking the electron deficient carbon atom.
Answer to Problem 7.76P
Compounds A, B, C and D are
Explanation of Solution
Sodium hydride on reaction with
Therefore, compound A is
The compound A acts like a nucleophile and attack on the electron deficient carbon atom of
Therefore, compound B is
Compound B on reaction with strong base
Therefore, compound C is
The compound C acts like a nucleophile and attack on the electron deficient carbon atom of
Therefore, compound D is
Compound A, B, C and D is
Want to see more full solutions like this?
Chapter 7 Solutions
ORGANIC CHEMISTRY W/ ACCESS >IC<
Additional Science Textbook Solutions
Chemistry
General, Organic, & Biological Chemistry
Introductory Chemistry (5th Edition) (Standalone Book)
Principles of General, Organic, Biological Chemistry
Chemistry: The Central Science (13th Edition)
Living By Chemistry: First Edition Textbook
- Devise a synthesis of muscalure, the sex pheromone of the common housefly, from acetylene and any other required reagents.arrow_forwardIdentify A, B, and C, three intermediates in the synthesis of the pain reliever and anesthetic fentanyl.arrow_forwardA key step in a synthesis of the antimalarial drug quinine involves an intramolecular nucleophilic substitution that converts A to B. Draw the structure of B and give the reagents needed to convert B to quinine.arrow_forward
- One step in the synthesis of the antihistamine fexofenadine involves acid-catalyzed hydration of the triple bond in A. Draw a stepwise mechanism for this reaction and explain why only ketone B is formed.arrow_forwardWhat product is formed when a solution of A to B is treated with mild base? This reaction is the first step in the synthesis of rosuvastatin (sold as a calcium salt under the trade name Crestor), a drug used to treat patients with high cholesterol.arrow_forwardWhat product is formed when a solution of A and B is treated with mild base? This reaction is the rst step in the synthesis of rosuvastatin (sold as a calcium salt under the trade name Crestor), a drug used to treat patients with high cholesterol.arrow_forward
- Identify M in the following reaction sequence used to prepare the antiulcer drug omeprazole (trade name Prilosec).arrow_forwardDevise a synthesis of muscalure, the sex pheromone of the common housey, from acetylene and any other required reagents.arrow_forwardTamoxifen is an estrogen receptor modulator that is used in the treatment of breast cancer. Provide the missing reagents and the structure of compound A in the synthesis of tamoxifen.arrow_forward
