
Concept explainers
In some nucleophilic substitutions under
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
KCTCS Organic Chemistry Value Edition (Looseleaf) - Text Only
Additional Science Textbook Solutions
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
General Chemistry: Atoms First
Essential Organic Chemistry (3rd Edition)
Chemistry: Atoms First
Organic Chemistry (9th Edition)
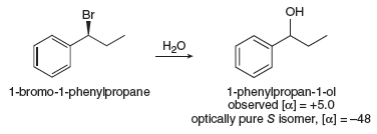
- The compound 3,4-dimethyl-hexan-3-ol of 3R, 4S configuration is treated with a concentrated HBr solution at room temperature. A mixture of two stereoisomers is obtained.If the reaction mixture above is heated, the appearance of several other compounds is observed. 1) Draw the different compounds obtained using the wedge-flywheel representation. 2) What is the majority product? Explain 3) Propose a modification of the experimental conditions in order to obtain the exclusive formation of these compounds obtained after heatingarrow_forwardIn some nucleophilic substitutions under SN1 conditions, complete racemization does not occur and a small excess of one enantiomer is present. For example, treatment of optically pure 1-bromo-1-phenylpropane with water forms 1- phenylpropan-1-ol. (a) Calculate how much of each enantiomer is present using the given optical rotation data. (b) Whichproduct predominates—the product of inversion or the product of retention of conguration? (c) Suggest an explanation for this phenomenon.arrow_forwardThe reaction of butan-2-ol with concentrated aqueous HBr goes with partial racemization, giving more inversion thanretention of configuration. Propose a mechanism that accounts for racemization with excess inversion.(b) Under the same conditions, an optically active sample of trans-2-bromocyclopentanol reacts with concentrated aqueous HBr to give an optically inactive product, (racemic) trans-1,2-dibromocyclopentane. Proposea mechanism to show how this reaction goes with apparently complete retention of configuration, yet withracemization. (Hint: Draw out the mechanism of the reaction of cyclopentene with Br2 in water to give thestarting material, trans-2- bromocyclopentanol. Consider how parts of this mechanism might be involved in thereaction with HBr.)arrow_forward
- The following alkene is treated with one equivalent of N-Bromosuccinimide in dichloromethane in the presence of light to give bromination product(s). Draw a line-angle formula for each product formed.arrow_forwardFive-membered aromatic heterocycles with one heteroatom undergo electrophilic substitutions preferentially at the α-position (C-2 and C-5) rather than at the β-position (C-3 and C-4). Explain?arrow_forward1-Chloro-1,2-diphenylethane can undergo E2 elimination to give either cis- or trans-1,2-diphenylethylene (stilbene). Draw Newman projections of the reactive conformations leading to both possible products, and suggest a reason why the trans alkene is the major product.arrow_forward
- Draw the products formed when both cis- and trans-but-2-ene are treated with OsO4, followed by hydrolysis with NaHSO3 + H2O. Explain how these reactions illustrate that syn dihydroxylation is stereospecific.arrow_forwardWhen the given alkene undergoes hydroboration followed by oxidation, which would be produced in regards to the products stereochemistry a. A mix of diastereomers b. A single enantiomer c. A racemic mixture d. An achiral productarrow_forwardThe reaction of (S)-2-bromopentane with potassium cyanide to yield 2-methylpentanenitrile (2-cyanopentane) occurs due to a nucleophilic substitution pathway. The reaction is 100% stereospecific. Please explain what this observation tells about the mechanism of the reaction.arrow_forward
- Q3. For the following reactions, predict the products, mechanism and name all the products obtained Catalytic hydrogenation of cycloalkenes. Reduction of cyclohexanone with Zinc amalgam (Zn/Hg alloy) in concentrated hydrochloric acid Addition of CH2I2 in an alkene in the presence of Zn-Cu 2-hexene + chlorine gas Halogenation of cyclohexane in the presence of light and catalyst Sulfonation of Cyclobutane with oleum Addition of HBr to methylcyclopropanearrow_forwardCycloheptatrienyl radical (C7H7∙) contains a cyclic, completely conjugated system of π electrons. Is it aromatic? Is it antiaromatic? Explain.arrow_forwardA8 Is there anyone who can provide this pericyclic rxn mechanism?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
