
EBK GENERAL CHEMISTRY: THE ESSENTIAL CO
7th Edition
ISBN: 8220106637203
Author: Chang
Publisher: YUZU
expand_more
expand_more
format_list_bulleted
Question
Chapter 7, Problem 7.91QP
Interpretation Introduction
Interpretation:
The ground-state electron configuration of selenium (
Concept introduction:
By using Aufbau principle, electrons starts to occupy from orbitals of lower energy level. Thus, the obtained lower energy configuration is known as ground-state electron configuration.
Aufbau principle:
- Electrons first occupy orbitals with lower energy than higher energy.
- An orbital can be occupied only by two electrons having opposite spins.
- Greater the number of parallel spins of electrons more stable the orbital. Each electron fills each orbital till it is half filled. This is known as Hund’s rule.
- The order of electrons filling in an atom is given as
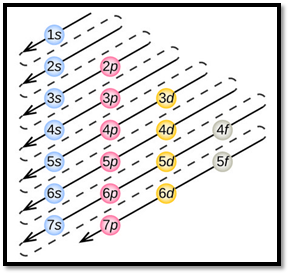
Figure 1
Pauli Exclusion Principle:
It states that in an atom no two electrons have same quantum numbers. That is two electrons in an atom can have same three quantum number (
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
EBK GENERAL CHEMISTRY: THE ESSENTIAL CO
Ch. 7.1 - Review of Concepts
What of the waves shown here...Ch. 7.1 - Prob. 1PECh. 7.1 - Prob. 2RCCh. 7.2 - Prob. 1PECh. 7.2 - Prob. 2PECh. 7.2 - Prob. 1RCCh. 7.3 - Prob. 1PECh. 7.3 - Prob. 1RCCh. 7.4 - Prob. 1PECh. 7.4 - Prob. 1RC
Ch. 7.5 - Prob. 1RCCh. 7.6 - Prob. 1RCCh. 7.7 - Prob. 1PECh. 7.7 - Prob. 2PECh. 7.7 - Prob. 1RCCh. 7.8 - Prob. 1PECh. 7.8 - Prob. 2PECh. 7.8 - Prob. 3PECh. 7.8 - Prob. 1RCCh. 7.9 - Prob. 1PECh. 7.9 - Prob. 1RCCh. 7 - Prob. 7.1QPCh. 7 - Prob. 7.2QPCh. 7 - Prob. 7.3QPCh. 7 - Prob. 7.4QPCh. 7 - Prob. 7.5QPCh. 7 - Prob. 7.6QPCh. 7 - Prob. 7.7QPCh. 7 - 7.8 (a) What is the frequency of tight having a...Ch. 7 - Prob. 7.9QPCh. 7 - Prob. 7.10QPCh. 7 - Prob. 7.11QPCh. 7 - 7.12 The SI unit of length is the meter, which...Ch. 7 - 7.13 What are photons? What role did Einstein's...Ch. 7 - Prob. 7.14QPCh. 7 - Prob. 7.15QPCh. 7 - Prob. 7.16QPCh. 7 - Prob. 7.17QPCh. 7 - Prob. 7.18QPCh. 7 - Prob. 7.19QPCh. 7 - Prob. 7.20QPCh. 7 - Prob. 7.21QPCh. 7 - Prob. 7.22QPCh. 7 - Prob. 7.23QPCh. 7 - Prob. 7.24QPCh. 7 - Prob. 7.25QPCh. 7 - Prob. 7.26QPCh. 7 - Prob. 7.27QPCh. 7 - Prob. 7.28QPCh. 7 - Prob. 7.29QPCh. 7 - Prob. 7.30QPCh. 7 - Prob. 7.31QPCh. 7 - Prob. 7.32QPCh. 7 - Prob. 7.33QPCh. 7 - Prob. 7.34QPCh. 7 - Prob. 7.35QPCh. 7 - Prob. 7.36QPCh. 7 - Prob. 7.37QPCh. 7 - Prob. 7.38QPCh. 7 - Prob. 7.39QPCh. 7 - Prob. 7.40QPCh. 7 - Prob. 7.41QPCh. 7 - 7.42 What is the de Broglie wavelength (in nm)...Ch. 7 - Prob. 7.43QPCh. 7 - Prob. 7.44QPCh. 7 - Prob. 7.45QPCh. 7 - Prob. 7.46QPCh. 7 - Prob. 7.47QPCh. 7 - Prob. 7.48QPCh. 7 - 7.49 Why is a boundary surface diagram useful in...Ch. 7 - Prob. 7.50QPCh. 7 - Prob. 7.51QPCh. 7 - Prob. 7.52QPCh. 7 - Prob. 7.53QPCh. 7 - Prob. 7.54QPCh. 7 - Prob. 7.55QPCh. 7 - Prob. 7.56QPCh. 7 - Prob. 7.57QPCh. 7 - 7.58 What is the difference between a 2px and a...Ch. 7 - Prob. 7.59QPCh. 7 - Prob. 7.60QPCh. 7 - Prob. 7.61QPCh. 7 - Prob. 7.62QPCh. 7 - Prob. 7.63QPCh. 7 - Prob. 7.64QPCh. 7 - 7.65 Make a chart of all allowable orbitals in the...Ch. 7 - 7.66 Why do the 3s, 3p, and 3d orbitals have the...Ch. 7 - Prob. 7.67QPCh. 7 - Prob. 7.68QPCh. 7 - Prob. 7.69QPCh. 7 - Prob. 7.70QPCh. 7 - Prob. 7.71QPCh. 7 - Prob. 7.72QPCh. 7 - Prob. 7.73QPCh. 7 - Prob. 7.74QPCh. 7 - Prob. 7.75QPCh. 7 - Prob. 7.76QPCh. 7 - Prob. 7.77QPCh. 7 - 7.78 Comment on the correctness of the following...Ch. 7 - Prob. 7.79QPCh. 7 - Prob. 7.80QPCh. 7 - Prob. 7.81QPCh. 7 - Prob. 7.82QPCh. 7 - Prob. 7.83QPCh. 7 - Prob. 7.84QPCh. 7 - Prob. 7.85QPCh. 7 - Prob. 7.86QPCh. 7 - Prob. 7.87QPCh. 7 - Prob. 7.88QPCh. 7 - Prob. 7.89QPCh. 7 - Prob. 7.90QPCh. 7 - Prob. 7.91QPCh. 7 - Prob. 7.92QPCh. 7 - Prob. 7.93QPCh. 7 - Prob. 7.94QPCh. 7 - 7.95 Identify the following individuals and their...Ch. 7 - Prob. 7.96QPCh. 7 - Prob. 7.97QPCh. 7 - Prob. 7.98QPCh. 7 - Prob. 7.99QPCh. 7 - 7.100 A laser is used in treating retina...Ch. 7 - 7.101 A 368-g sample of water absorbs infrared...Ch. 7 - Prob. 7.102QPCh. 7 - Prob. 7.103QPCh. 7 - Prob. 7.104QPCh. 7 - Prob. 7.105QPCh. 7 - Prob. 7.106QPCh. 7 - Prob. 7.107QPCh. 7 - Prob. 7.108QPCh. 7 - Prob. 7.109QPCh. 7 - Prob. 7.110QPCh. 7 - Prob. 7.111QPCh. 7 - 7.112 An atom moving at its root-mean-square speed...Ch. 7 - Prob. 7.113QPCh. 7 - Prob. 7.114QPCh. 7 - Prob. 7.115QPCh. 7 - Prob. 7.116QPCh. 7 - Prob. 7.117SPCh. 7 - Prob. 7.118SPCh. 7 - Prob. 7.119SPCh. 7 - Prob. 7.120SPCh. 7 - 7.121 According to Einstein’s special theory of...Ch. 7 - Prob. 7.122SPCh. 7 - Prob. 7.123SPCh. 7 - Prob. 7.124SPCh. 7 - Prob. 7.125SPCh. 7 - 7.126 The wave function for the 2s orbital in the...Ch. 7 - Prob. 7.127SPCh. 7 - Prob. 7.128SPCh. 7 - Prob. 7.129SP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
Periodic Properties of Elements | Chemistry | IIT-JEE | NEET | CBSE | Misostudy; Author: Misostudy;https://www.youtube.com/watch?v=L26rRWz4_AI;License: Standard YouTube License, CC-BY
Periodic Trends: Electronegativity, Ionization Energy, Atomic Radius - TUTOR HOTLINE; Author: Melissa Maribel;https://www.youtube.com/watch?v=0h8q1GIQ-H4;License: Standard YouTube License, CC-BY