
Concept explainers
(a)
Interpretation:
The formula mass of ascorbic acid has to be calculated.
Concept introduction:
Formula mass is defined as addition of
(a)
Answer to Problem 7.AE
The formula mass of ascorbic acid is
Explanation of Solution
To calculate: The formula mass of ascorbic acid.
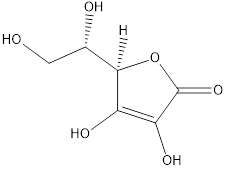
The molecular formula of ascorbic acid is
The formula mass is determined as follows,
(b)
Interpretation:
The molarity of
Concept introduction:
Molarity can be defined as the moles of solute (in grams) to the volume of the solution (in litres). The molarity of a solution can be given by the formula,
(b)
Answer to Problem 7.AE
The molarity of
Explanation of Solution
To calculate: The molarity of
The molecular weight of ascorbic acid is
The number of moles of ascorbic acid is calculated as
There are equal number of moles for both ascorbic acid and
(c)
Interpretation:
The mass percentage of ascorbic acid in given tablet has to be calculated.
Concept introduction:
The mass percentage of compound can be calculated by taking the calculated mass of the substance to the total mass of the sample whole multiplied by 100.
The mass percent can be calculated with formula,
Molarity can be defined as the moles of solute (in grams) to the volume of the solution (in litres). The molarity of a solution can be given by the formula,
(c)
Answer to Problem 7.AE
The mass percentage of ascorbic acid in given tablet is
Explanation of Solution
To calculate: The mass percentage of ascorbic acid in given tablet.
The number of moles of
There are equal number of moles for both ascorbic acid and
The mass of ascorbic acid is derived from molecular weight of ascorbic acid
The mass percent of ascorbic acid in given tablet is
Want to see more full solutions like this?
Chapter 7 Solutions
Quantitative Chemical Analysis
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





