
(a)
Interpretation:
The mechanism for the reaction of 2-butyne with one equivalent of bromine should be given.
Concept Introduction:
Addition of halides to
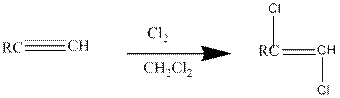
If excess halides are present, a second addition reaction also occurs.
(b)
Interpretation:
The configuration of the product obtained in the above reaction should be identified.
Concept Introduction:
Addition of halides to alkynes: Halogens like chlorine and bromine adds to the alkyne compounds. The general form of the reaction is given below.

If excess halides are present, a second addition reaction also occurs.
Trending nowThis is a popular solution!

Chapter 7 Solutions
Modified Mastering Chemistry with Pearson eText -- Standalone Access Card -- for Organic Chemistry (8th Edition)
- Is the reaction of 2-butene with HBr regioselective?arrow_forward2. In the bromination reactions, what is the function of CCl4? Why can it fulfil its role?3. Bromination proceeds by either free radical substitution or electrophillic addition. Based on Table 3, which mechanism is followed by alkanes? by alkenes? by alkynes?4. For which hydrocarbon type is light necessary for bromination to take place?arrow_forwardWhat are the colors of bromine and potassium permanganate? Write the chemical equations involved in the reaction of cyclohexene with the following: a.) Br2 b.) cold KMnO4arrow_forward
- Phineas and Ferbs, two brothers who enjoy vacations, doing fun things every summer. This summer the brothers and their friends carry out an organic synthesis with an unknown compound (L1) that contains 52% Carbon, 6% Hydrogen and 42% bromine, this compound (L1) is treated with magnesium in ether to obtain L2 , which reacts violently with D2O for 1-methyl cyclohexene with a deuterium atom in the methyl group (L3). The L2 reaction is treated with acetone followed by hydrolysis to give L4. Heating L4 with concentrated sulfuric acid gives L5, which decolors the bromine, obtaining L6. L5 undergoes hydrogenation with excess hydrogen and platinum as a catalyst giving rise to isobutyl cyclohexane. Determine the structures of compounds L1 through L6.arrow_forwardDraw the carbocation you'd expect from the reaction of 2-chloro-3-methylbutane with AlCl3?arrow_forwardReaction of HBr with 2-methylpropene yields 2-bromopropane. What is the structure of the carbocation intermediate formed during the reaction? Show the mechanism of the reaction.arrow_forward
- Why does bromine's color disappear when Br2 is added to an alkene sample?arrow_forwardReaction of hbr with 2-methylpropene yields 2 bromo 2 methylpropane. What is the structure of the carbocation formed during the reaction? Show the mechanism of reaction.arrow_forward1) What is the major product for the reaction of 3-bromo1-butene and HBr? Give the name of the product. 2) For this product, how many chiral carbon(s) are there in the structure?3) How many stereoisomers could we write for this product? 4) Write the Fischer Projections of all stereoisomers. (attach a file or other ways you could show your work)arrow_forward
- 2. In the bromination reactions, what is the function of CCl4? Why can it fulfil its role?3. Bromination proceeds by either free radical substitution or electrophillic addition. Based on Table 3, which mechanism is followed by alkanes? by alkenes? by alkynes?4. For which hydrocarbon type is light necessary for bromination to take place?5. What is the function of light in the bromination reaction? Why are alkenes and alkynes not included as samples?arrow_forwardOf the four possible cis,trans isomers, one is formed in over 85% yield. Q.) Which is the structure of the isomer formed in 85% yield? How do you account for its formation? Create a model to help you make this prediction.arrow_forwardChlorination of 2-butanone yields two isomeric products, each having the molecular formula C4H7ClO. (a) What are these two compounds? (b) Write structural formulas for the enol intermediates that lead to each of these compounds. (c) Using curved arrows, show the flow of electrons in the reaction of each of the enols with Cl2.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

