
Interpretation:
The
Concept Introduction:
Acid Catalysed addition of water: When water is added to alkyne in the presence of an acid, the product formed will be an enol. Enol contains a double bond and a
If a carbonyl group is bonded to two alkyl groups, it is called as a ketone. The enol formed in the acid catalysed addition of water will be easily converted into a ketone.
Conversion of terminal
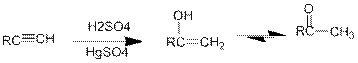
Want to see the full answer?
Check out a sample textbook solution
Chapter 7 Solutions
Organic Chemistry - MasteringChemistry
- Prepare the following substances from aldehydes or ketonesarrow_forwardDraw line structures of the following compounds and the product you would obtain from the reduction of each.(a) Isopropyl methyl ketone (b) p-Hydroxybenzaldehyde(c) 2-Methylcyclopentanonearrow_forwardConverting aldehyde to hydroxyketone using a-silyloxyketonesarrow_forward
- Describe how the target molecule (butanone) can be synthesized in a high yield from butane.arrow_forwardWhich is a dehydrating agent in the preparation of Pyroxylin USP? H3BO3 HCl H2SO4 HNO3arrow_forwardwhat functional groups are present in each of the reagents and products in the reaction of acid-catalysed esterification of octanoic acid with 1-propanol (Fischer esterification)?arrow_forward
- is the carbon from the C=O of an aldehyde more reactive or the carbon of the C=N-Imine and why? Dontarrow_forwardWrite the name of the product that results when cyclopentanone is treated with Pt and hydrogen gas.arrow_forwarddescribe how steric and electronic effects influence the postion of equilibrium when the electrophilic center of an aldehyde or ketone is under nucleophilic attack.arrow_forward

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

