
Organic Chemistry - MasteringChemistry
8th Edition
ISBN: 9780134074665
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 7, Problem 41P
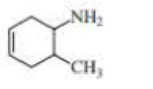
What is each compound’s systematic name?
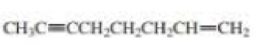
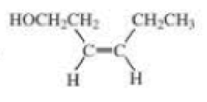
- a. CH3CH2C≡CCH2CH2C≡CH
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Which group in each pair is assigned the higher priority?
a. – CH3, – CH2CH3
b. – I, – Br
c. – H, – D
d. – CH2Br, – CH2CH2Br
e. – CH2CH2Cl, – CH2CH(CH3)2
f. – CH2OH, – CHO
Rank the following groups in order of decreasing priority.
a.−COOH, −H, −NH2, −OH
b.−H, −CH3, −Cl, −CH2Cl
c. −CH2CH3, −CH3, −H, −CH(CH3)2
d.−CH=CH2, −CH3, −C≡CH, −H
(a) Draw all constitutional isomers formed by monochlorination of each alkane with Cl2 and hν. (b) Draw the major monobromination product formed by heating each alkane with Br2.
Chapter 7 Solutions
Organic Chemistry - MasteringChemistry
Ch. 7.1 - Prob. 1PCh. 7.1 - Name the following:Ch. 7.1 - What is the molecular formula for a monocyclic...Ch. 7.1 - Draw the condensed and skeletal structures for...Ch. 7.1 - Draw the structure and give the common and...Ch. 7.2 - Prob. 6PCh. 7.2 - Name the following:Ch. 7.3 - What orbitals are used to form the carbon-carbon ...Ch. 7.4 - Prob. 9PCh. 7.4 - Why does cis-2-butene have a higher boiling point...
Ch. 7.6 - Prob. 12PCh. 7.6 - Prob. 13PCh. 7.7 - Prob. 14PCh. 7.7 - Which alkyne should be used for the synthesis of...Ch. 7.7 - Prob. 16PCh. 7.8 - Prob. 17PCh. 7.8 - Only one alkyne forms an aldehyde when it...Ch. 7.9 - Describe the alkyne you should start with and the...Ch. 7.9 - Prob. 20PCh. 7.10 - Prob. 21PCh. 7.10 - Prob. 22PCh. 7.10 - Prob. 23PCh. 7.10 - Rank the following from strongest base to weakest...Ch. 7.10 - Prob. 26PCh. 7.12 - Prob. 28PCh. 7 - What is the major product obtained from the...Ch. 7 - Draw a condensed structure for each of the...Ch. 7 - A student was given the structural formula of...Ch. 7 - Prob. 32PCh. 7 - What is each compounds systematic name?Ch. 7 - What reagents should be used to carry out the...Ch. 7 - Prob. 35PCh. 7 - Draw the mechanism for the following reaction:Ch. 7 - Prob. 37PCh. 7 - Prob. 38PCh. 7 - What is the major product of the reaction of 1 mol...Ch. 7 - Answer Problem 39, parts a-b, using 2-butyne as...Ch. 7 - What is each compounds systematic name? a....Ch. 7 - What is the molecular formula of a hydrocarbon...Ch. 7 - a. Starting with 3-methyl 1-butyne, how can you...Ch. 7 - Prob. 44PCh. 7 - Which of the following pairs are keto-enol...Ch. 7 - Prob. 46PCh. 7 - Do the equilibria of the following acid-base...Ch. 7 - What is each compounds systematic name?Ch. 7 - What stereoisomers are obtained when 2-butyne...Ch. 7 - Prob. 50PCh. 7 - Draw the keto tautomer for each of the following:Ch. 7 - Show how each of the following compounds can be...Ch. 7 - A chemist is planning to synthesize 3-octyne by...Ch. 7 - Prob. 54PCh. 7 - What stereoisomers are obtained from the following...Ch. 7 - Prob. 56PCh. 7 - Prob. 57PCh. 7 - Prob. 58PCh. 7 - Show how the following compound can be prepared...Ch. 7 - Prob. 60P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Each H↔H eclipsing interaction in ethane costs about 4.0 kJ/mol. How many such interactions are percent in cyclopropane? What fraction of the overall 115 kJ/mol (27.5 kcal/mol) strain energy of cyclopropane is due to torsional strain.arrow_forwardDraw the eight constitutional isomers having the molecular formula C5H11Cl. a.Give the IUPAC name for each compound (ignoring R and S designations). b.Classify each alkyl halide as 1°, 2°, or 3°. c.Label any stereogenic centers. d.For each constitutional isomer that contains a stereogenic center, draw all possible stereoisomers, and label each stereogenic center as R or S.arrow_forwardDraw the eight constitutional isomers having the molecular formula C5H11Cl.a. Give the IUPAC name for each compound (ignoring R and S designations).b. Classify each alkyl halide as 1°, 2°, or 3°.c. Label any stereogenic centers.d. For each constitutional isomer that contains a stereogenic center, draw all possible stereoisomers, and label each stereogenic center as R or S.arrow_forward
- Which carbocation is more stable?H2C=C+H OR HC=C+ b HC= C+ OR CH3C+H2arrow_forwardRank the following groups in order of decreasing priority. a. – COOH, – H, – NH2, – OH b. – H, – CH3, – Cl, – CH2CI c. -CH2CH3, -CH3, -H, -CH(CH3)2 d. – CH = CH2, – CH3, – C ≡ CH, – Harrow_forwardA is a toxin produced by the poisonous seaweed Chlorodesmis fastigiata. (a) Label each alkene that exhibits stereoisomerism as E or Z. (b) Draw a stereoisomer of A that has all Z double bonds.arrow_forward
- Draw the following compounds: a) 6-[1-chloropropyl]-5-methyl-1-decyne b) 2-bromo-3-methyl-3,5-octadienec) 4,6,6-trimethylcyclooctened) 2-Bromobicyclo[3.3.1]nonane e) 2-Methylbicyclo[2.2.2]octane f) 1,2-Dichlorocyclohexeneg) 4,5-Dibromo-1-pentenearrow_forwarda. How many stereoisomers are formed from the reaction of cyclohexene with NBS?b. How many stereoisomers are formed from the reaction of 3-methylcyclohexene with NBS?arrow_forwardDraw all stereoisomers formed when each alkene is treated with CHCl3 and KOC(CH3)3.arrow_forward
- Draw the structure of each compound. sodium cyclopentadienidearrow_forwardDraw the products formed when CH3CH2C=C Na+ reacts with each compound. a. CH3CH2CH2Br b.(CH3)2CHCH2CH2Cl c.(CH3CH2)3CCl d.BrCH2CH2CH2CH2OH e. ethylene oxide followed by H2O f.propene oxide followed by H2Oarrow_forward(a) What product(s) are formed when the E isomer of C6H5CH = CHC6H5 is treated with Br2, followed by one equivalent of KOH? Label the resulting alkene(s) as E or Z. (b) What product(s) are formed when the Z isomer of C6H5CH = CHC6H5 is subjected to the same reaction sequence? (c) How are the compounds in parts (a) and (b) related to each other?arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you

 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning


Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Nomenclature: Crash Course Chemistry #44; Author: CrashCourse;https://www.youtube.com/watch?v=U7wavimfNFE;License: Standard YouTube License, CC-BY