
Concept explainers
(a)
Interpretation:
Molar mass of the compound has to be determined.
Concept Introduction:
Ideal gas Equation:
Any gas is described by using four terms namely pressure, volume, temperature and the amount of gas. Thus combining three laws namely Boyle’s, Charles’s Law and Avogadro’s Hypothesis the following equation could be obtained. It is referred as ideal gas equation.
Here,
n is the moles of gas
P is the Pressure
V is the Volume
T is the Temperature
R is the gas constant
Molar mass can be determined using the given equation,
Here, n is the number of moles.
M is the Molar mass.
m is the Mass.
(a)
Answer to Problem 116QRT
Molar mass of the compound is
Explanation of Solution
Given information is shown below,
Number of moles of the compound is determined using Ideal gas equation as given,
Molar mass of the compound is determined as follows,
Molar mass of the compound is
(b)
Interpretation:
Empirical formula and molecular formula for the compound has to be given.
Concept Introduction:
Empirical Formula:
Empirical formula of a compound is the simplest formula which provides the lowest positive whole number ratio of atoms that exists in the compound. The molecular formula of a compound can either be same as the empirical formula or a multiple of it.
Percent composition:
Percent composition is nothing but providing the mass percent of each element present in the compound.
(b)
Answer to Problem 116QRT
Empirical formula of the compound is
Molecular formula for the compound is
Explanation of Solution
Given data:
The percent composition of the ingredient in the compound is given below:
Empirical Formula:
Let us assume that the mass of the compound is
From the percent composition given, calculate the number of moles of each element in the ingredient.
Mole calculation for carbon
Mole calculation for hydrogen,
Mole calculation of fluorine,
On dividing all the three numbers by the smallest integer
Empirical formula of the compound was determined.
Hence, the empirical formula will be
Molecular formula can also be written as
Empirical mass is calculated as follows,
Molar mass of the compound is
Molecular formula for the compound can be determined using the given formula,
Molecular formula for the compound is
(c)
Interpretation:
Lewis structure of each of the isomer of compound has to be determined.
Concept-Introduction:
Lewis structure
Electron dot structure also known as Lewis dot structure represents the number of valence electrons of an atom or constituent atoms bonded in a molecule. Each dot corresponds to one electron.
(c)
Explanation of Solution
Molecular formula for the compound is
Three isomers (two cis and one trans) are possible for the compound having this molecular formula.
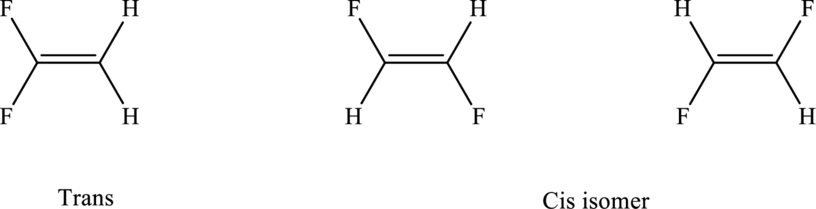
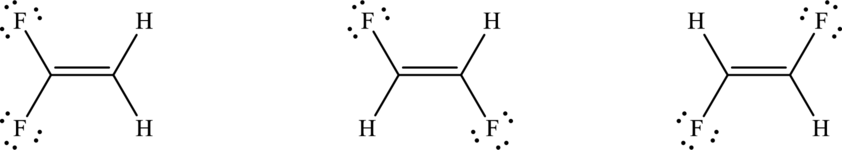
The Lewis electron dot structure for given molecules are determined by first drawing the skeletal structure for the given molecules, then the total number of valence electrons for all atoms present in the molecules are determined.
The next step is to subtract the electrons present in the total number of bonds present in the skeletal structure of the molecule with the total valence electrons such that considering each bond contains two electrons with it.
Finally, the electrons which got after subtractions have to be equally distributed considering each atom contains eight electrons in its valence shell.
Draw Lewis structure of the compound:
Outer valence electrons of Carbon Oxygen and Fluorine are four, six and seven respectively.
Here, one double bond is required to complete the complete the octets of all the atoms.
After the distribution of electrons, both fluorine atoms get two lone pair of electrons.
The Lewis structure of each of the isomer of compound follows as,
Want to see more full solutions like this?
Chapter 8 Solutions
Chemistry: The Molecular Science
- You have two pressure-proof steel cylinders of equal volume, one containing 1.0 kg of CO and the other containing 1.0 kg of acetylene, C2H2. (a) In which cylinder is the pressure greater at 25 C? (b) Which cylinder contains the greater number of molecules?arrow_forwardHow does hydraulic fracturing differ from previously used techniques for the recovery of natural gas from the earth?arrow_forwardA sample of a smoke stack emission was collected into a 1.25-L tank at 752 mm Hg and analyzed. The analysis showed 92% CO2, 3.6% NO, 1.2% SO2, and 4.1% H2O by mass. What is the partial pressure exerted by each gas?arrow_forward
- Given that a sample of air is made up of nitrogen, oxygen, and argon in the mole fractions 0.78 N2, 0.21 O2, and 0.010 Ar, what is the density of air at standard temperature and pressure?arrow_forwardOne of the chemical controversies of the nineteenth century concerned the element beryllium (Be). Berzelius originally claimed that beryllium was a trivalent element (forming Be3+ ions) and that it gave an oxide with the formula Be2O3. This resulted in a calculated atomic mass of 13.5 for beryllium. In formulating his periodic table, Mendeleev proposed that beryllium was divalent (forming Be2+ ions) and that it gave an oxide with the formula Be2O3. This assumption gives an atomic mass of 9.0. In 1894, A. Combes (Comptes Rendus 1894, p. 1221) reacted beryllium with the anion C5H7O2and measured the density of the gaseous product. Combess data for two different experiments are as follows: I II Mass 0.2022 g 0.2224 g Volume 22.6 cm3 26.0 cm3 Temperature 13C 17C Pressure 765.2 mm Hg 764.6 mm If beryllium is a divalent metal, the molecular formula of the product will be Be(C5H7O2)2; if it is trivalent, the formula will be Be(C5H7O2)3. Show how Combess data help to confirm that beryllium is a divalent metal.arrow_forwardPyruvic acid, HC3H3O3, is involved in cell metabolism. It can be assayed for (that is, the amount of it determined) by using a yeast enzyme. The enzyme makes the following reaction go to completion: HC3H3O3(aq)C2H4O(aq)+CO2(g) If a sample containing pyruvic acid gives 21.2 mL of carbon dioxide gas, CO2, at 349 mmHg and 30C, how many grams of pyruvic acid are there in the sample?arrow_forward
- The Mount Pinatubo volcano eruption in 1991 released an estimated 1.82 x 1013g of SO2 into the atmosphere. If the gas had an average temperature of -17.0 C and filled the troposphere, whose approximate volume is 8 x 1021L, what is the approximate partial pressure of SO2 caused by the eruption?arrow_forwardIn the discussion on the composition of air, mention is made of the fact that water vapor may have a concentration as high as 40,000 ppm. Calculate the partial pressure exerted by water vapor at this concentration. Assume that this represents a situation with 100% humidity. What temperature would be needed to achieve this value? (See Appendix G.)arrow_forward
 Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning,
Physical ChemistryChemistryISBN:9781133958437Author:Ball, David W. (david Warren), BAER, TomasPublisher:Wadsworth Cengage Learning, Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





