
Concept explainers
(a)
Interpretation: The name of the given alcohol needs to be determined, the stereochemistry (if present) needs to be indicated and it needs to be labeled as primary, secondary and tertiary alcohol.
Concept Introduction: An alcohol can be classified as primary, secondary and tertiary deepening on the number of hydrogen atoms attached to the C atom to which the hydroxyl group is attached. If the number of H atoms are two, the alcohol is primary in nature, if there is only 1 H atom attached, then the alcohol is secondary and if there is no H atom attached to the C atom then the alcohol is tertiary.
(a)
Explanation of Solution
The given organic compound is as follows:
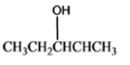
The name of the compound is 2-butanol.
Here, hydroxyl group is attached to the secondary carbon thus, it is a secondary alcohol.
(b)
Interpretation: The name of the given alcohol needs to be determined, the stereochemistry (if present) needs to be indicated and it needs to be labeled as primary, secondary and tertiary alcohol.
Concept Introduction: An alcohol can be classified as primary, secondary and tertiary deepening on the number of hydrogen atoms attached to the C atom to which the hydroxyl group is attached. If the number of H atoms are two, the alcohol is primary in nature, if there is only 1 H atom attached, then the alcohol is secondary and if there is no H atom attached to the C atom then the alcohol is tertiary.
(b)
Explanation of Solution
The given organic compound is as follows:
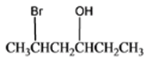
The name of the above compound is 5-bromo-3-hexanol.
Here, the hydroxyl group is at secondary carbon thus, it is a secondary alcohol.
(c)
Interpretation: The name of the given alcohol needs to be determined, the stereochemistry (if present) needs to be indicated and it needs to be labeled as primary, secondary and tertiary alcohol.
Concept Introduction: An alcohol can be classified as primary, secondary and tertiary deepening on the number of hydrogen atoms attached to the C atom to which the hydroxyl group is attached. If the number of H atoms are two, the alcohol is primary in nature, if there is only 1 H atom attached, then the alcohol is secondary and if there is no H atom attached to the C atom then the alcohol is tertiary.
(c)
Explanation of Solution
The given organic compound is as follows:
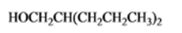
The name of the compound is 2-propyl-1-pentanol.
Now, there is hydroxyl group at primary position thus, it is a primary alcohol.
(d)
Interpretation: The name of the given alcohol needs to be determined, the stereochemistry (if present) needs to be indicated and it needs to be labeled as primary, secondary and tertiary alcohol.
Concept Introduction: An alcohol can be classified as primary, secondary and tertiary deepening on the number of hydrogen atoms attached to the C atom to which the hydroxyl group is attached. If the number of H atoms are two, the alcohol is primary in nature, if there is only 1 H atom attached, then the alcohol is secondary and if there is no H atom attached to the C atom then the alcohol is tertiary.
(d)
Explanation of Solution
The given organic compound is as follows:
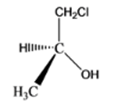
The name of the compound is (S)-1-chloro-2-propanol. There is a hydroxyl group at secondary carbon thus, it is a secondary alcohol.
(e)
Interpretation: The name of the given alcohol needs to be determined, the stereochemistry (if present) needs to be indicated and it needs to be labeled as primary, secondary and tertiary alcohol.
Concept Introduction: An alcohol can be classified as primary, secondary and tertiary deepening on the number of hydrogen atoms attached to the C atom to which the hydroxyl group is attached. If the number of H atoms are two, the alcohol is primary in nature, if there is only 1 H atom attached, then the alcohol is secondary and if there is no H atom attached to the C atom then the alcohol is tertiary.
(e)
Explanation of Solution
The given organic compound is as follows:
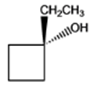
The name of the above compound is 1-ethylcyclobutanol. The hydroxyl group is at tertiary carbon thus, it is a tertiary alcohol.
(f)
Interpretation: The name of the given alcohol needs to be determined, the stereochemistry (if present) needs to be indicated and it needs to be labeled as primary, secondary and tertiary alcohol.
Concept Introduction: An alcohol can be classified as primary, secondary and tertiary deepening on the number of hydrogen atoms attached to the C atom to which the hydroxyl group is attached. If the number of H atoms are two, the alcohol is primary in nature, if there is only 1 H atom attached, then the alcohol is secondary and if there is no H atom attached to the C atom then the alcohol is tertiary.
(f)
Explanation of Solution
The given organic compound is as follows:
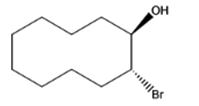
The name of the compound is (1R,2R)-2bromocyclodecanol.
In the above compound, the hydroxyl group is at secondary position thus, this is a secondary alcohol.
(g)
Interpretation: The name of the given alcohol needs to be determined, the stereochemistry (if present) needs to be indicated and it needs to be labeled as primary, secondary and tertiary alcohol.
Concept Introduction: An alcohol can be classified as primary, secondary and tertiary deepening on the number of hydrogen atoms attached to the C atom to which the hydroxyl group is attached. If the number of H atoms are two, the alcohol is primary in nature, if there is only 1 H atom attached, then the alcohol is secondary and if there is no H atom attached to the C atom then the alcohol is tertiary.
(g)
Explanation of Solution
The given organic compound is as follows:
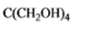
The name of the compound is 2, 2-bis(hydroxylmethyl)propane-1,3-
(h)
Interpretation: The name of the given alcohol needs to be determined, the stereochemistry (if present) needs to be indicated and it needs to be labeled as primary, secondary and tertiary alcohol.
Concept Introduction: An alcohol can be classified as primary, secondary and tertiary deepening on the number of hydrogen atoms attached to the C atom to which the hydroxyl group is attached. If the number of H atoms are two, the alcohol is primary in nature, if there is only 1 H atom attached, then the alcohol is secondary and if there is no H atom attached to the C atom then the alcohol is tertiary.
(h)
Explanation of Solution
The given organic compound is as follows:
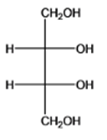
The name of the molecule is (2S,3R)-2,3-dihydroxybutane-1,2-diol. The hydroxyl group at first and fourth carbon atoms is primary and the other two hydroxyl groups are at second and third carbon atoms which are secondary.
(i)
Interpretation: The name of the given alcohol needs to be determined, the stereochemistry (if present) needs to be indicated and it needs to be labeled as primary, secondary and tertiary alcohol.
Concept Introduction: An alcohol can be classified as primary, secondary and tertiary deepening on the number of hydrogen atoms attached to the C atom to which the hydroxyl group is attached. If the number of H atoms are two, the alcohol is primary in nature, if there is only 1 H atom attached, then the alcohol is secondary and if there is no H atom attached to the C atom then the alcohol is tertiary.
(i)
Explanation of Solution
The given organic compound is as follows:
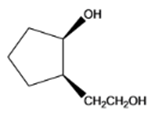
The name of the compound is cis-2-(2-hydroxylethyl)cyclopentanol. Here, the hydroxyl group is at secondary carbon atom of the cyclopentyl ring and the other hydroxyl group is attached to the primary carbon atom.
(j)
Interpretation: The name of the given alcohol needs to be determined, the stereochemistry (if present) needs to be indicated and it needs to be labeled as primary, secondary and tertiary alcohol.
Concept Introduction: An alcohol can be classified as primary, secondary and tertiary deepening on the number of hydrogen atoms attached to the C atom to which the hydroxyl group is attached. If the number of H atoms are two, the alcohol is primary in nature, if there is only 1 H atom attached, then the alcohol is secondary and if there is no H atom attached to the C atom then the alcohol is tertiary.
(j)
Explanation of Solution
The given compound is as follows:
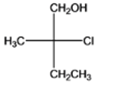
The name of the compound is (2R)-2-chloro-2-methyl-1-butanol. The hydroxyl group is at primary carbon atom thus, it is primary alcohol.
(k)
Interpretation: The name of the given alcohol and its all possible stereoisomers needs to be determined and it needs to be labeled as primary, secondary and tertiary alcohol.
Concept Introduction: An alcohol can be classified as primary, secondary and tertiary deepening on the number of hydrogen atoms attached to the C atom to which the hydroxyl group is attached. If the number of H atoms are two, the alcohol is primary in nature, if there is only 1 H atom attached, then the alcohol is secondary and if there is no H atom attached to the C atom then the alcohol is tertiary.
(k)
Explanation of Solution
The given structure is as follows:
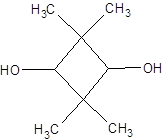
Since, there is no chiral center thus, there will be no possible stereoisomers.
The name of the given alcohol is 2, 2, 4, 4-tetramethylcyclobutane-1, 3-diol. Both the −OH groups are on the C atom with 1 H atom each thus, both are secondary alcohols.
Want to see more full solutions like this?
Chapter 8 Solutions
ORGANIC CHEM F/UT (LOOSELEAF)
- There are 17 possible alkene isomers with the formula C6H12. Draw structures of the five isomers in which the longest chain has six carbon atoms, and give the name of each. Are any of these isomers chiral? (There are also eight isomers in which the longest chain has five carbon atoms, and four isomers in which the longest chain has four carbon atoms. How many can you find?)arrow_forwardClassify the following alcohols as primary, secondary, or tertiary: a. b. CH3CH2CH2CH2OH c.arrow_forwardArrange these compounds in order of increasing boiling point (values in C are 42, 24, 78, and 118). (a) CH3CH2OH (b) CH3OCH3 (c) CH3CH2CH3 (d) CH3COOHarrow_forward
- Draw a structural formula for an alkene with the indicated molecular formula that gives the compound shown as the major product. Note that more than one alkene may give the same compound as the major product. CHa | C5Hw + H,O CH3CCH2CH3 OH CHa I C5H10 + Br2 » CH3CHCHCH, Br Br 7H12 + HC1 oearrow_forwardWhat functional group distinguishes each of the following hydrocarbon derivatives? a. halohydrocarbons b. alcohols c. ethers d. aldehydes e. ketones f. carboxylic acids g. esters h. amines Give examples of each functional group. What prefix or suffix is used to name each functional group? What are the bond angles in each? Describe the bonding in each functional group. What is the difference between a primary, secondary, and tertiary alcohol? For the functional groups in ah, when is a number required to indicate the position of the functional group? Carboxylic acids are often written as RCOOH. What does COOH indicate and what does R indicate? Aldehydes are sometimes written as RCHO. What does CHO indicate?arrow_forwardDraw structural formulas for all possible carbocations formed by the reaction of each alkene with HC1. Label each carbocation as primary, secondary, or tertiary. ch3 I ch3ch,c=chch3 ch3ch2ch=chch3arrow_forward
- Which of the following pairs of cycloalkanes represent structural isomers? a. b. c. d.arrow_forwardThere are four cis,trans isomers of 2-isopropyl-5-methylcyclohexanol: (a) Using a planar hexagon representation for the cyclohexane ring, draw structural formulas for the four cis,trans isomers. (b) Draw the more stable chair conformation for each of your answers in part (a). (c) Of the four cis,trans isomers, which is most stable? (Hint: If you answered this part correctly, you picked the isomer found in nature and given the name menthol.)arrow_forward
 Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning
Introduction to General, Organic and BiochemistryChemistryISBN:9781285869759Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar TorresPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning





