
Concept explainers
Oxymercuration
Concerns about mercury’s toxicity have led to decreased use of mercury-based reagents in
Among the synthetically useful reactions of
salts with organic compounds, the most familiar is a two-stage procedure for alkene hydration called oxymercuration–demercuration. Its application in the conversion of
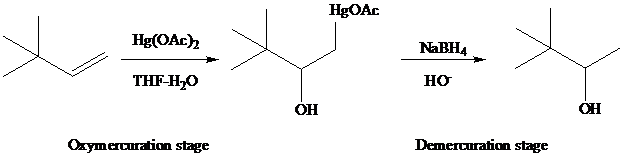
The reaction is performed in two operations, the first of which is oxymercuration. In this stage the alkene is treated with
of the alkene. The oxygen of water, one of the components in the
solvent mixture, bonds to
to
From the overall reaction, we see that oxymercuration–demercuration
1. accomplishes hydration of the double bond in accordance with Markovnikov’s rule, and
2. carbocation rearrangements do not occur.
Additional information from stereochemical studies with other
3. anti addition of
and
characterizes the oxymercuration stage, and
4. the replacement of
c by H in the demercuration stage is not stereospecific.
The structure of the intermediate in oxymercuration has received much attention and can be
approached by considering what is likely to happen when the electrophile
reacts with the double bond of an alkene.
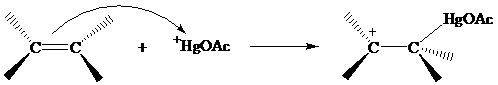
Recall from Section
that electrons in bonds that are
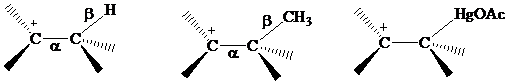
The electrons in a
or
electrons, making
stabilization by hyperconjugation more effective for
or
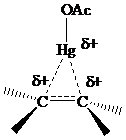
The problems that follow explore various synthetic aspects of oxymercuration–demercuration.
Experimental procedures sometimes vary depending on the particular transformation. The source of the electrophile may be a mercury(II) salt other than
Oxymercuration–demercuration of allyl alcohol gives
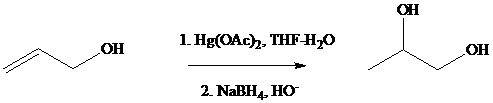
Under the same conditions, however,
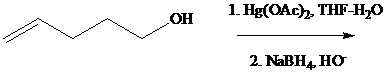
What is the most reasonable structure for the product of this reaction?
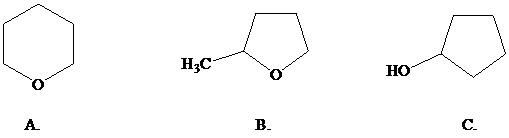
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
ORGANIC CHEM BUNDLE >BI<
- . Each of the following alcohols or alkyl halides has been subjected to either acid-catalyzed dehydration or base dehydrohalogenation respectively and yields a mixture of two or more isomeric alkenes. Identify the alkenes in each case, and predict which one is the major product on the basis of either Zaitsev rule, stability of the alkene or rearrangement. Give the IUPAC name, including stereochemistry where necessary, of each organic reactant and product and state whether it is the E1 or E2 productarrow_forwardCompound P (C2H4) which is an alkene undergoes reaction with HCl to produce compound Q (C2H5Cl). Reaction of compound Q with benzene in the presence of AlCl3 as catalyst produces compound R. Then, nitration of compound R in the presence ofnH2SO4 produces two compounds, S and T. But when compound R is reacted with a hot acidified solution of alkaline KMnO4 gives compound U. Deduce the structure of compounds P to U.arrow_forwardAs the molecular weight of alkenes increases, the boiling points also increase. Which ofthe following factors is best associated to this trend? *Dipole interactionGeometric isomerismStructural isomerismSurface areaA radical substitution reaction is primarily observed in the following reactions. Whichreaction is it? *Oxidation of but-2-eneChlorination of butaneBoiling of hex-3-eneCombustion of methaneWhich among the following compounds has the most electronegative carbon in itsstructure? *ButyneBenzeneBenzaldehydeCyclobutanearrow_forward
- A synthetic organic molecule, G, which contains both aldehyde and ether functional groups, is subjected to a series of reactions in a multi-step synthesis pathway. In the first step, G undergoes a Wittig reaction, leading to the formation of an alkene, H. Subsequently, H is treated with an ozone (O3) reagent followed by a reducing agent in an ozonolysis reaction, resulting in the formation of two different products, I and J. Considering the functional groups present in G and the nature of the reactions involved, what are the most probable structures or functional groups present in products I and J? A. I contains a carboxylic acid group, and J contains an aldehyde group. B. I contains a ketone group, and J contains an alcohol group. C. I and J both contain aldehyde groups. D. I contains an ester group, and J contains a ketone group. Don't use chat gpt.arrow_forwardProvide a reasonable synthetic strategy for the synthesis of a racemic mixture of (1R,2R) and (1S,2S)-2-bromo-1-methylcyclopentanol from the compound shown below... Provide the bond line structure for the major organic products obtained in each step of the proposed strategyarrow_forwardAs per the guidelines, three sub-parts will be answered, please help me with these three parts j, k lloudon 6th edition organic chemistry For each of the following reactions,provide the major product(s) or the necessary reagents to perform the chemical transformations indicated. If no reaction takes place, write NRarrow_forward
- Given that five isomeric compounds (A, B, C, D, E) with the molecular formula C5H10O were subjected to chemical tests after undergoing Clemmensen reduction compounds A,B, and C all yielded n-pentane, the results of these tests are provided in the table below. Can you draw the structures of compounds A to E based on the outcomes of the chemical tests?arrow_forwardReaction of -pinene with borane followed by treatment of the resulting trialkylborane with alkaline hydrogen peroxide gives the following alcohol. Of the four possible cis,trans isomers, one is formed in over 85% yield. (a) Draw structural formulas for the four possible cis,trans isomers of the bicyclic alcohol. (b) Which is the structure of the isomer formed in 85% yield? How do you account for its formation? Create a model to help you make this prediction.arrow_forwardThe compound whose structure is shown here is acetyl acetone. It exists in two forms:the enol form and the keto form The molecule reacts with OH–to form an anion, [CH3COCHCOCH3] (often abbreviatedacac–for acetylacetonate ion). One or the most interesting aspects of this anion is thatone or more of them can react with transition metal cations to give stable, highlycolored compounds (a) Are the keto and enol forms of acetylacetone resonance forms? Explain youranswer.(b) What is the hybridization or each atom (except H) in the enol form? What changesin hybridization occur when it is transformed into the keto form?(c) What are the electron-pair geometry and molecular geometry around each C atomin the keto and enol forms? What changes in geometry occur when the keto formchanges to the enol form?(d) Draw three possible resonance structures for the acac–ion.(e) Is cis-trans isomerism possible in either the enol or the keto form of acetylacetone?(f) Is the enol form of acetylacetone polar?…arrow_forward
- Suggest reagents and reaction conditions for each of the following reactionsarrow_forwardthe organic compound 2-heptanone, belonging to the ketone family, is responsible for the strong penetrating odor in Roquefort cheeses. Starting from acetylene as the starting reagent, propose a synthesis line with the reaction mechanisms involved for the synthetic obtaining of 2-heptanone and use it as a food additive in analogous cheeses.arrow_forwardStarting with benzene and using any other necessary reagents of your choice, what are the possible syntheses for the following compound?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

