
Interpretation:
Series of chain initiation, propagation and termination steps for the reaction and estimate its heat of reaction has to be proposed.
Concept introduction:
Halogenation of
Radical chain reaction:
Initiation reaction: 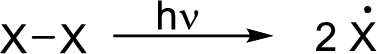
Chain propagation: 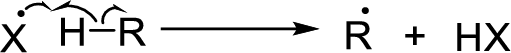
Chain termination:
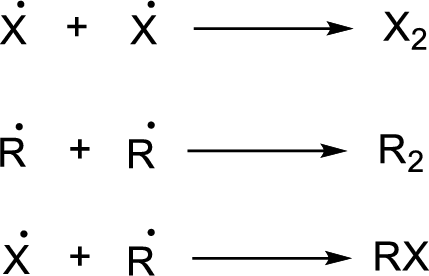
Heat of reaction for a radical reaction can be identified form bond dissociation enthalpy.
It is a change in enthalpy of a homolysis reaction at absolute zero where a molecule is broken down into two free radicals.
Trending nowThis is a popular solution!

Chapter 8 Solutions
Organic Chemistry
- There are two isomeric cyclohexa-1,4-diene products when toluene undergoes the Birch reduction (see Problem 25.24). (a) Draw the mechanism that leads to the formation of the major product. (b) Will the Birchreduction of toluene occur faster or slower than the Birch reduction of benzene itself? Hint: Is –CH3 an electron-donating or an electron-withdrawing group?arrow_forwardPredict the products of the following reduction reactions, including stereochemistry where needed. If the reaction product is racemic, indicate that by writing “racemic”. please explain stepsarrow_forwardCan you please predict the MAJOR product or provide the reagents/conditions for the following reactions? Please indicate racemic if it is and if there is no reaction, please specify that as well. ?arrow_forward
- Fill in the missing reactant along with optically active or racemic name and what reaction it goes by E1, E2, SN1, OR SN2arrow_forwardDraw the structure of the expected organic product(s) formed in the following reactions including correct sine echemistry. If the product is racemic write racemic or draw both isomers. Assume all reagents listed are present in excess unless otherwise noted. If no reaction occurs, state "No Reaction".arrow_forwardEach of the following line structures can be described as a bicyclononane except for.arrow_forward
- (c) The following reaction shows the electrophillc addition reaction between an alkene compound with hydrogen chloride, HCI. Tindak balas berikut menunjukkan tindak balas penambahan elektrofilik antara sebatian alkena dengan hidrogen klorida, HCI. HCI Major product Draw the mechanism for the formation of major product. Lukis mekanisma bagi pembentukan hasil utama berikut.arrow_forwardPredict the major product(s) for each of the following thermal electrocyclic reactions. Justify your answer with MO theory. Heat Heat Heatarrow_forwardConsider the tetracyclic compound with rings labeled A–D. (a) Which ring is the most reactive in electrophilic aromatic substitution? (b) Which ring is the least reactive in electrophilic aromatic substitution?arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
