
Concept explainers
(a)
Interpretation:
The mechanism for the given reaction has to be given.
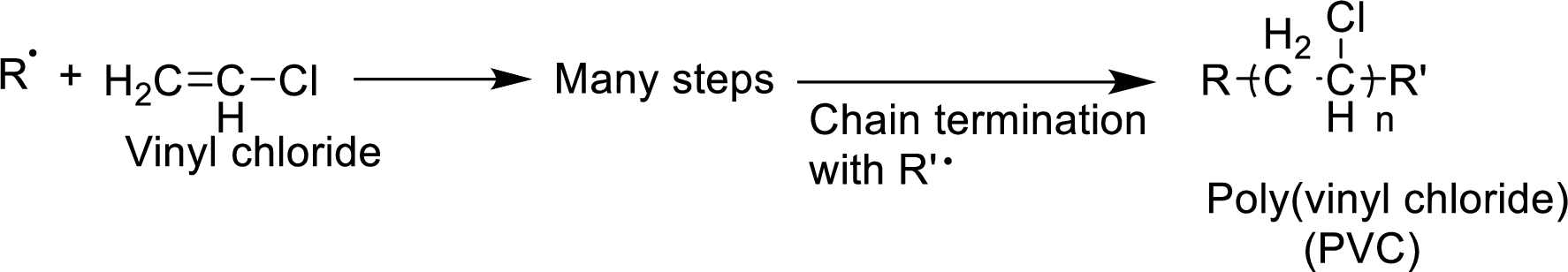
Concept Introduction:
The
- 1. Chain initiation
- 2. Chain propagation and
- 3. Chain termination.
Chain initiation occurs by the formation of radical from one of the monomer units. Propagation occurs by the reaction of the radicals with molecules. Chain termination occurs by neutralization of radicals.
(b)
Interpretation:
The mechanism for the formation of poly(styrene) from styrene has to be given and at which end of the styrene double bond, the
Concept Introduction:
The polymers are formed from the repetition monomer units. The polymerization process occurs in three steps.
- 1. Chain initiation
- 2. Chain propagation and
- 3. Chain termination.
Chain initiation occurs by the formation of radical from one of the monomer units. Propagation occurs by the reaction of the radicals with molecules. Chain termination occurs by neutralization of radicals.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
Organic Chemistry
- Mm.31. Subject :- Chemistryarrow_forward6. Dacron is the brand name for the polymer that is made from ethane-1,2-diol and benzene-1,4-dicarboxylic acid. (a) When the two monomers combine, what type of reaction do they undergo and what molecule is eliminated? [1] (b) What is the name for the linkages that join the monomers together? [1] (c) Draw the monomers and the polymer of the reaction to create Dacron. [3]arrow_forward(a) Hard contact lenses, which first became popular in the 1960s, were made by polymerizing methyl methacrylate [CH; =C(CH,)CO,CH3] to form poly(methyl methacrylate) (PMMA). Draw the structure of PMMA. (b) More comfortable softer contact lenses introduced in the 1970s were made by polymerizing hydroxyethyl methacrylate [CH2=C{CH)CO,CH,CH2OH] to form poly(hydroxyethyl methacrylate) (poly-HEMA). Draw the structure of poly-HEMA. Since neither polymer allows oxygen from the air to pass through to the retina, newer contact lenses that are both comfortable and oxygen-permeable have now been developed.arrow_forward
- A major use of Cl2 is in the manufacture of vinyl chloride,the monomer of poly(vinyl chloride). The two-step sequence forformation of vinyl chloride is depicted below.(a) Write a balanced equation for each step. (b) Write the overallequation. (c) What type of organic reaction is shown in step 1?(d) What type of organic reaction is shown in step 2? (e) If eachmolecule depicted in the initial reaction mixture represents0.15 mol of substance, what mass (in g) of vinyl chloride forms?arrow_forwardDetermine the functionality of the following monomer CH₂ CH2 –NH2 HOOC–CH2 – CH2-C-CH2-C=CH, in a reaction that produces ester linkages. in a reaction that produces amide linkages; (i) (ii) (iii) in a free radical addition reaction Give an example of monomer that can be used in each of the above reactions.arrow_forward(b) Now write a short note on the possible structures of polystyrene as formed from styrene (phenylethene, Ph-CH=CH2). You should define the terms tacticity, isotactic, and atactic.arrow_forward
- (a) A sample of linear polyethylene is dissolved in a large excess of xylene at 130 C, and dilute solution is then slowly cooled. After a while, a fine white suspension is obtained. What does this suspension consist of? (b) If the suspension is filtered and dried, then heated to 170C, and cooled slowly to room temperature, a different type of structure is obtained. Compare and contrast the two different structures.arrow_forwardIn a recent year, the United States produced 6.26 × 109kg1,2-dichloroethane and 3.73 × 109 kg vinyl chloride.Assuming that all significant quantities of vinyl chloridewere produced from 1,2-dichloroethane, what fraction ofthe 1,2-dichloroethane production went into making vinylchloride? What mass of hydrogen chloride was generatedas a by-product?arrow_forward(1) Explain the following polymerization mechanism by showing propagation reactions with the catalyst three times (draw chemical structures of the intermediates of the first, second, and third reaction with the monomer and catalysts). LnW=CHR' tanda (2) Why is HT-3-hexyl polythiophene better in conductivity than other regioisomers? Explain the reasons from the intramolecular and intermolecular aspects, respectively.arrow_forward
- Kindly answer question a(i) & barrow_forwardPhotochemical chlorination of 2,2,4-trimethylpentane gives four isomeric monochlorides. (a) Write structural formulas for these four isomers. (b) The two primary chlorides make up 65% of the monochloride fraction. Assuming that all the primary hydrogens in 2,2,4-trimethylpentane are equally reactive, estimate the percentage of each of the two primary chlorides in the product mixture.arrow_forward4. Outline the following synthesis (a). Butane to 2-butanol (b). 1,2-dibromopropane from 2-propanol (c). 1-butanol to 2-butanol (d). Propane to Propenearrow_forward
 Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning
Principles of Modern ChemistryChemistryISBN:9781305079113Author:David W. Oxtoby, H. Pat Gillis, Laurie J. ButlerPublisher:Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning
Chemistry: The Molecular ScienceChemistryISBN:9781285199047Author:John W. Moore, Conrad L. StanitskiPublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning




