
(a)
Interpretation:
The equilibria and charge and mass balances needed to find the composition of
Concept introduction:
Charge balance:
The overall positive charges in solution equals the overall negative charges in solution.
Mass balance:
The amount of all the species in a solution containing a particular atom (or a group of atoms) must equal the amount of that atom (or group) delivered to the solution.
(a)
Explanation of Solution
Given information,
Pertinent reactions are:
Write the charge balance equation
Write the mass balance equation
(b)
Interpretation:
The concentration of species in
Concept introduction:
Charge balance:
The overall positive charges in solution equals the overall negative charges in solution.
Mass balance:
The amount of all the species in a solution containing a particular atom (or a group of atoms) must equal the amount of that atom (or group) delivered to the solution.
(b)
Explanation of Solution
Given information,
Pertinent reactions are:
Write the charge balance equation
Write the mass balance equation
First neglect activity coefficients. Make the following substitutions in the charge balance equation:
Equate the expressions for
Now substitute
We can now substitute for all terms in the charge balance using
Charge balance:
Rearrange the above equation
The spreadsheet for the calculation of species concentrations is given in figure 1
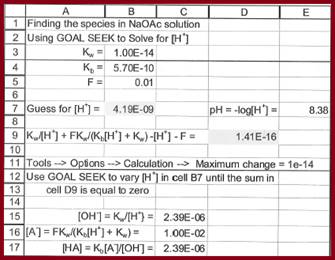
Figure 1
The concentrations are given below
The value of ionic strength is
The value of pH is
Fraction of hydrolysis
Want to see more full solutions like this?
Chapter 8 Solutions
Quantitative Chemical Analysis
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





