
Concept explainers
(a) Draw all products formed by treatment of each dibromide (A and B) with one equivalent of
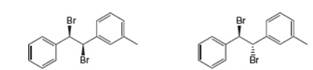
A B
(a)
Interpretation: All products formed by the treatment of the given dibromide (A and B) with one equivalent of
Concept introduction: The two-step unimolecular elimination reaction that favors the removal of a HX substituent and the formation of a carbocation intermediate takes place in its first step. In the second step of the reaction, the carbocation forms a double bond. This type of reaction is termed E1 elimination reaction.
The one-step bimolecular elimination reaction that favors the removal of a proton by a base from carbon adjacent to the leaving group that results in the formation of a carbocation is termed as
Answer to Problem 8.68P
The products that formed by the treatment of the given dibromide A with one equivalent of
Explanation of Solution
The reaction of dibromide A with one equivalent of
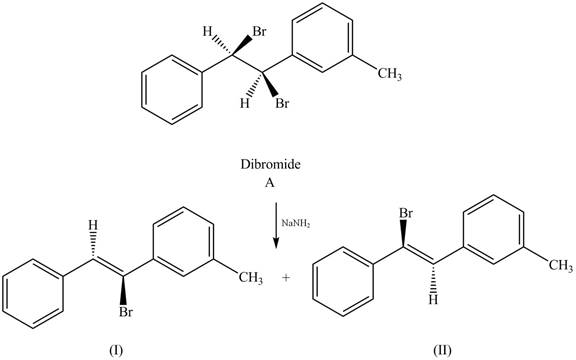
Figure 1
In this reaction, the given dihalide undergoes elimination reaction in the presence of one equivalent of the strong base,
The reaction of dibromide B with one equivalent of
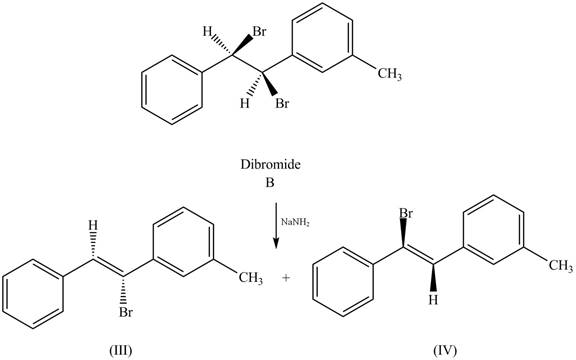
Figure 2
In this reaction, the given dihalide undergoes elimination reaction in the presence of one equivalent of the strong base,
The products that formed by the treatment of the given dibromide A with one equivalent of
(b)
Interpretation: The labeling of the pairs of diastereomers and constitutional isomers are to be shown.
Concept introduction: Diastereomers compounds are not mirror images of each other and are different in configuration at one or more stereocentres.
The isomers which have same molecular formula but different connectivity of atoms are constitutional isomers.
Answer to Problem 8.68P
The labeling of the pairs of diastereomers is,
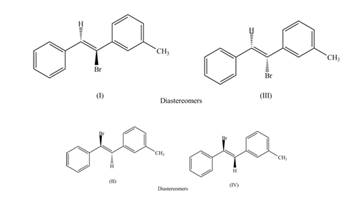
The labeling of the pairs of constitutional isomers is,
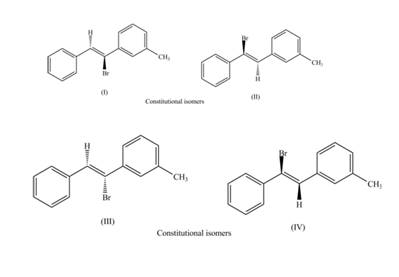
Explanation of Solution
The first pair of diastereomers is shown as,
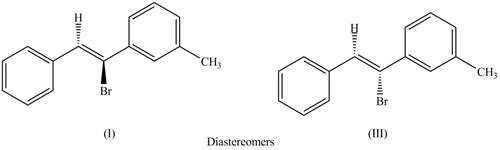
Figure 3
Thus, the products (I) and (III) are diastereomers that are not mirror images of each other and are differ in configuration.
The second pair of diastereomers is shown as,
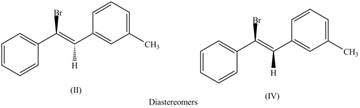
Figure 4
Thus, the products (II) and (IV) are diastereomers that are not mirror images of each other and are differ in configuration. (I), (II) and (III),(IV) are constitutional isomers as they differ only in arrangement of bonds.
The first pair of constitutional isomers is shown as,
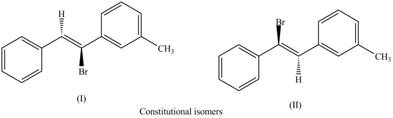
Figure 5
Thus, the products (I) and (II) are constitutional isomers as they possess different arrangement of bonds.
The first pair of constitutional isomers is shown as,
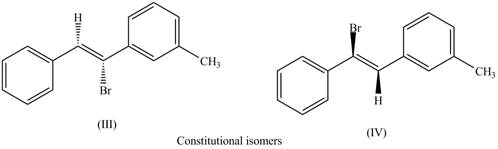
Figure 6
Thus, the products (III) and (IV) are constitutional isomers as they possess different arrangement of bonds.
The labeling of the pairs of diastereomers is shown in Figure 3 and Figure 4. The labeling of the pairs of constitutional isomers is shown in Figure 5 and Figure 6.
Want to see more full solutions like this?
Chapter 8 Solutions
ORG.CHEMISTRY W/ACCESS+MODEL KIT PKG
Additional Science Textbook Solutions
Chemistry & Chemical Reactivity
Fundamentals of Heat and Mass Transfer
EBK INTRODUCTION TO CHEMISTRY
Chemistry by OpenStax (2015-05-04)
Living by Chemistry
General Chemistry: Atoms First
- Draw all products formed by treatment of each dibromide (A and B) with one equivalent of NaNH2arrow_forwardHow is compound A related to compounds B–E? Choose from enantiomers, diastereomers, constitutional isomers, or identical molecules.arrow_forward(a) Are compounds B–D identical to or an isomer of A? (b) Give the IUPAC name for A.arrow_forward
- Draw compounds a, b, and c.arrow_forward(a) What product(s) are formed when the E isomer of C6H5CH = CHC6H5 is treated with Br2, followed by one equivalent of KOH? Label the resulting alkene(s) as E or Z. (b) What product(s) are formed when the Z isomer of C6H5CH = CHC6H5 is subjected to the same reaction sequence? (c) How are the compounds in parts (a) and (b) related to each other?arrow_forward(a) What is the major alkene formed when A is dehydrated with H2SO4? (b) What is the major alkene formed when A is treated with POCl3 and pyridine? Explain why the major product is different in these reactions.arrow_forward
- The shrub ma huang (Section 5.4A) contains two biologically activestereoisomers—ephedrine and pseudoephedrine—with two stereogeniccenters as shown in the given structure. Ephedrine is one component ofa once-popular combination drug used by body builders to increaseenergy and alertness, whereas pseudoephedrine is a nasaldecongestant.a.) Draw the structure of naturally occurring (−)-ephedrine, which has the1R,2S configuration.b.) Draw the structure of naturally occurring (+)-pseudoephedrine, whichhas the 1S,2S configuration.c.) How are ephedrine and pseudoephedrine related?d.) Draw all other stereoisomers of (−)-ephedrine and (+) pseudoephedrine, and give the R,S designation for all stereogeniccenters.e.) How is each compound drawn in part (d) related to (−)-ephedrine?arrow_forwardDraw the products formed when p-methylaniline (p-CH3C6H4NH2) istreated with following reagent. Part (b), then CH3COCl, AlCl3arrow_forwardDraw the products formed when A or B is treated with each reagent. In some cases, no reaction occurs. [1] C6H5Li (excess); [2] H2Oarrow_forward
- 5. Draw the structure for compounds B and Carrow_forwardProvide the missing starting material, reactant or product. Show appropriate stereochemistry. a) b)arrow_forwardHydrogenation of alkene A with D2 in the presence of Pd-C affords a single product B. Keeping this result in mind, what compound is formed when A is treated with each reagent: (a) mCPBA; (b) Br2, H2O followed by base? Explain these results.arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





