
Principles of General Organic & Biological Chemistry
2nd Edition
ISBN: 9780077633721
Author: Janice Smith
Publisher: Mcgraw-hill Higher Education (us)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8, Problem 8.90AP
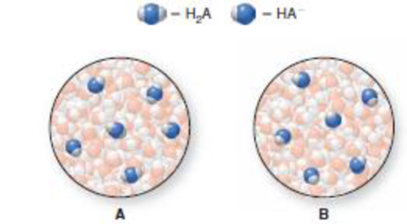
Consider a weak acid H2A and its conjugate base HA−. Which diagram represents a buffer? Explain your choice.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
The acidity of a solution is measured by its pH.
If HT| represents the concentration of hydrogen ions (in moles/liter) in the solution,
the pH is defined by pH = – log H+
Based on careful measurements and calculations, a chemist examines two solutions and asserts:
"The hydrogen ion concentration of Solution A
is 37.93% greater than the hydrogen ion concentration of Solution B."
If the pH of solution B is 4.36, determine the pH of Solution A.
Report your answer to two decimal places.
Solution A has pH equal to
Number
- (Report to the nearest 0.01)
Chemistry
The label of an energy drink states that it contains approximately 80 mg of caffeine (C8H10N4O2) per 250 mL serving. If the caffeine is a weak base with a basicity constant of 2.5 × × 10-4, what is the pH of the drink? (Only caffeine is considered to contribute to the pH of the drink.)
The acidity of a solution is measured by its pH.
If Ht represents the concentration of hydrogen ions (in moles/liter) in the solution,
the pH is defined by pH = – log H+
Based on careful measurements and calculations, a chemist examines two solutions and asserts:
"The hydrogen ion concentration of Solution A
is 158 times greater than
the hydrogen ion concentration of Solution B."
If the pH of solution B is 3.4, determine the pH of Solution A.
Report your answer to one decimal place.
Solution A has pH equal to
Number
(Report to the nearest 0.1)
Chapter 8 Solutions
Principles of General Organic & Biological Chemistry
Ch. 8.1 - Name each acid: (a) HF; (b) HNO3; (c) HCN.Ch. 8.1 - Prob. 8.2PCh. 8.1 - Which of the following species can be BrnstedLowry...Ch. 8.1 - Which of the following species can be BrnstedLowry...Ch. 8.1 - Classify each reactant as a BrnstedLowry acid or...Ch. 8.2 - Draw the conjugate acid of each species: (a) H2O;...Ch. 8.2 - Draw the conjugate base of each species: (a) H2S;...Ch. 8.2 - Draw the structure of the conjugate base of each...Ch. 8.2 - Label the acid and the base and the conjugate acid...Ch. 8.2 - Ammonia, NH3, is amphoteric. (a) Draw the...
Ch. 8.2 - When ascorbic acid (vitamin C, molecular formula...Ch. 8.3 - Prob. 8.12PCh. 8.3 - Prob. 8.13PCh. 8.3 - Prob. 8.14PCh. 8.3 - Prob. 8.15PCh. 8.4 - Calculate the value of [OH] from the given [H3O+]...Ch. 8.4 - Calculate the value of [H3O+] from the given [OH]...Ch. 8.4 - Calculate the value of [H3O+] and [OH] in each...Ch. 8.5 - Convert each H3O+ concentration to a pH value. a....Ch. 8.5 - What H3O+ concentration corresponds to each pH...Ch. 8.5 - Prob. 8.21PCh. 8.5 - Prob. 8.22PCh. 8.6 - Write a balanced equation for each acidbase...Ch. 8.6 - Prob. 8.24PCh. 8.6 - Prob. 8.25PCh. 8.6 - Write a balanced equation for the reaction of...Ch. 8.7 - Prob. 8.27PCh. 8.7 - Prob. 8.28PCh. 8.8 - Prob. 8.29PCh. 8.8 - Prob. 8.30PCh. 8 - Draw the structure of the conjugate base of each...Ch. 8 - Draw the structure of the conjugate base of each...Ch. 8 - (a) Which of the following represents a strong...Ch. 8 - Prob. 8.34UKCCh. 8 - Identify the acid, base, conjugate acid, and...Ch. 8 - Prob. 8.36UKCCh. 8 - Prob. 8.37UKCCh. 8 - Prob. 8.38UKCCh. 8 - Prob. 8.39UKCCh. 8 - Prob. 8.40UKCCh. 8 - If a urine sample has a pH of 5.90, calculate the...Ch. 8 - Prob. 8.42UKCCh. 8 - Prob. 8.43UKCCh. 8 - Prob. 8.44UKCCh. 8 - Consider a buffer prepared from the weak acid HNO2...Ch. 8 - Prob. 8.46UKCCh. 8 - Prob. 8.47APCh. 8 - Prob. 8.48APCh. 8 - Prob. 8.49APCh. 8 - Prob. 8.50APCh. 8 - Prob. 8.51APCh. 8 - Prob. 8.52APCh. 8 - Draw the conjugate base of each acid. a. HNO2 b....Ch. 8 - Draw the conjugate base of each acid. a. H3O+ b....Ch. 8 - Prob. 8.55APCh. 8 - Prob. 8.56APCh. 8 - Prob. 8.57APCh. 8 - Like H2O, H2PO4 is amphoteric. (a) Draw the...Ch. 8 - Prob. 8.59APCh. 8 - Prob. 8.60APCh. 8 - Prob. 8.61APCh. 8 - Prob. 8.62APCh. 8 - Prob. 8.63APCh. 8 - Prob. 8.64APCh. 8 - Prob. 8.65APCh. 8 - Prob. 8.66APCh. 8 - Prob. 8.67APCh. 8 - Prob. 8.68APCh. 8 - Calculate the value of [OH] from the given [H3O+]...Ch. 8 - Calculate the value of [OH] from the given [H3O+]...Ch. 8 - Calculate the value of [H3O+] from the given [OH]...Ch. 8 - Prob. 8.72APCh. 8 - Prob. 8.73APCh. 8 - Calculate the pH from each H3O+ concentration...Ch. 8 - Prob. 8.75APCh. 8 - Prob. 8.76APCh. 8 - What are the concentrations of H3O+ and OH in...Ch. 8 - Prob. 8.78APCh. 8 - Prob. 8.79APCh. 8 - Prob. 8.80APCh. 8 - Prob. 8.81APCh. 8 - Prob. 8.82APCh. 8 - Prob. 8.83APCh. 8 - Prob. 8.84APCh. 8 - Prob. 8.85APCh. 8 - Prob. 8.86APCh. 8 - Prob. 8.87APCh. 8 - Prob. 8.88APCh. 8 - Consider a weak acid H2A and its conjugate base...Ch. 8 - Consider a weak acid H2A and its conjugate base...Ch. 8 - Prob. 8.91APCh. 8 - Prob. 8.92APCh. 8 - Prob. 8.93APCh. 8 - Prob. 8.94APCh. 8 - The optimum pH of a swimming pool is 7.50....Ch. 8 - A sample of rainwater has a pH of 4.18. (a)...Ch. 8 - Prob. 8.97APCh. 8 - Prob. 8.98APCh. 8 - Prob. 8.99APCh. 8 - Explain why a lake on a bed of limestone is...Ch. 8 - Prob. 8.101CP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Write two BrnstedLowry acid-base reactions and show how they represent proton-transfer reactions.arrow_forwardA scientist has synthesized a diprotic organic acid, H2A, with a molar mass of 124.0 g/mol. The acid must be neutralized (forming the potassium salt) for an important experiment. Calculate the volume of 0.221 M KOH that is needed to neutralize 24.93 g of the acid, forming K2A.arrow_forwardClassify each of the following as a strong or weak acid or base. a NH3 b HCNO c Mg(OH)2 d HClO3arrow_forward
- How many mEq of HCO3 are present in a solution that also contains 75 mEq of Na+, 83 mEq K+, 10 mEq Ca2+, and 153 mEq Cl?arrow_forwardUsing the diagrams shown in Problem 10-117, which of the solutions would have the greatest buffer capacity, that is, greatest protection against pH change, when the following occurs? a. A strong acid is added to the solution. b. A strong base is added to the solution.arrow_forwardOne half liter (500. mL) of 2.50 M HCl is mixed with 250. mL of 3.75 M HCl. Assuming the total solution volume after mixing is 750. mL, what is the concentration of hydrochloric acid in the resulting solution? What is its pH?arrow_forward
- Most naturally occurring acids are weak acids. Lactic acid is one example. CH3CH(OH)CO2H(s)+H2O(l)H3O+(aq)+CH3CH(OH)CO2(aq) If you place some lactic acid in water, it will ionize to a small extent, and an equilibrium will be established. Suggest some experiments to prow that this is a weak acid and that the establishment of equilibrium is a reversible process.arrow_forwardWrite chemical equations showing the individual proton-transfer steps that occur in aqueous solution for each of the following acids. a. H2C2O4 (oxalic acid) b. H2C4H4O6 (tartaric acid)arrow_forwardTwo strategies are also followed when solving for the pH of a base in water. What is the strategy for calculating the pH of a strong base in water? List the strong bases mentioned in the text that should be committed to memory. Why is calculating the pH of Ca(OH)2 solutions a little more difficult than calculating the pH of NaOH solutions? Most bases are weak bases. The presence of what element most commonly results in basic properties for an organic compound? What is present on this element in compounds that allows it to accept a proton? Table 13-3 and Appendix 5 of the text list Kb values for some weak bases. What strategy is used to solve for the pH of a weak base in water? What assumptions are made when solving for the pH of weak base solutions? If the 5% rule fails, how do you calculate the pH of a weak base in water?arrow_forward
- Consider a buffer solution that consists of two separate components: the weak acid, CH3COOH(aq), and its conjugate base, CH3COO¯ (aq) (added as NaCH3COO). Which component will react with H+(aq) from a strong acid? Write the net ionic equation for the reaction that occurs when H+(aq) is added to the buffered solution. Which component will react with OH-(aq) from a strong base? Write the net ionic equation for the reaction that occurs when OH-(aq) is added to the buffered solution.arrow_forwardCranberry juice contains significant amounts of malic acid (H2C4H4O5), a diprotic acid, which accounts for its low pH. If one cup of cranberry juice contains 3.667 g of malic acid, calculate how many extra strength TUMS tablets would be needed to completely neutralize the malic acid (if extra-strength tablets contains 7.50 x 102 mg of CaCO3). Show your calculations in full.arrow_forwardThe acidity of a solution is measured by its pH. If Ht represents the concentration of hydrogen ions (in moles/liter) in the solution, the pH is defined by pH - log Ht| Based on careful measurements and calculations, a chemist examines two solutions and asserts: "The hydrogen ion concentration of Solution A is 65.86% greater than the hydrogen ion concentration of Solution B." If the pH of solution B is 10.00, determine the pH of Solution A. Report your answer to two decimal places. Solution A has pH equal to Number (Report to the nearest 0.01)arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER
Living By Chemistry: First Edition TextbookChemistryISBN:9781559539418Author:Angelica StacyPublisher:MAC HIGHER Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781337399074Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning
Chemistry & Chemical ReactivityChemistryISBN:9781133949640Author:John C. Kotz, Paul M. Treichel, John Townsend, David TreichelPublisher:Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:9781559539418
Author:Angelica Stacy
Publisher:MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:9780078746376
Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:Glencoe/McGraw-Hill School Pub Co

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:Cengage Learning
General Chemistry | Acids & Bases; Author: Ninja Nerd;https://www.youtube.com/watch?v=AOr_5tbgfQ0;License: Standard YouTube License, CC-BY