
(a)
Interpretation:
The concentration of species in
Concept introduction:
Charge balance:
The overall positive charges in solution equals the overall negative charges in solution.
Mass balance:
The amount of all the species in a solution containing a particular atom (or a group of atoms) must equal the amount of that atom (or group) delivered to the solution.
(a)
Explanation of Solution
Given information,
Write the charge balance equation
Write the mass balance equation
Mass balance is equivalent to charge balance.
The equilibrium constant expression is given by
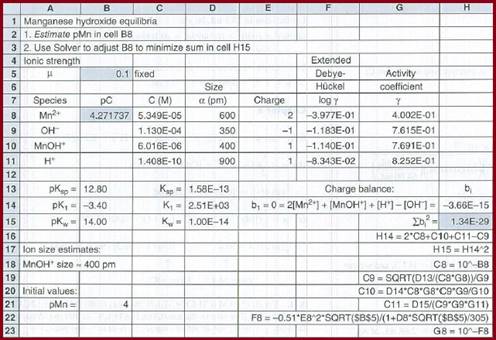
Figure 1
The concentration of all the species is given below:
(b)
Interpretation:
The concentration of species in
Concept introduction:
Charge balance:
The overall positive charges in solution equals the overall negative charges in solution.
Mass balance:
The amount of all the species in a solution containing a particular atom (or a group of atoms) must equal the amount of that atom (or group) delivered to the solution.
(b)
Explanation of Solution
Given information,
Write the charge balance equation
Write the mass balance equation
Mass balance is equivalent to charge balance.
The equilibrium constant expression is given by
The spreadsheet is the same as part (a), except that cell
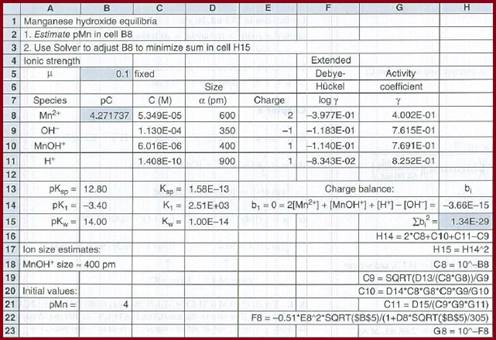
Figure 1
The concentration of the all species was found as
(c)
Interpretation:
The solubility of
(c)
Explanation of Solution
Given information,
The solubility of
Reason for the observation:
The solubility of
The quotient is calculated by
The value of quotient is
Want to see more full solutions like this?
Chapter 8 Solutions
QUANTIT.CHEM..(LL)-W/WEBASSIGN(6 MONTH)
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





