
ORGANIC CHEMISTRY 345 WSU >IP<
15th Edition
ISBN: 9781269916264
Author: Pearson
Publisher: PEARSON C
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 8.10, Problem 16P
Interpretation Introduction
Interpretation:
From the compounds given below A, B, C, D the one that can be protonated without destroying its aromaticity has to be identified.
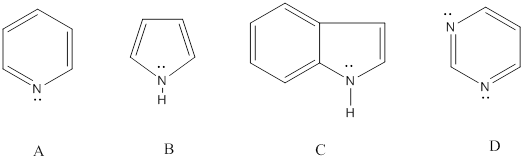
Concept Introduction:
An organic compound is said to be
A compound is aromatic if it is cyclic, planar and completely conjugated having
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
ORGANIC CHEMISTRY 345 WSU >IP<
Ch. 8.1 - Prob. 1PCh. 8.1 - Prob. 2PCh. 8.4 - Prob. 3PCh. 8.5 - Prob. 5PCh. 8.6 - a. Predict the relative bond lengths of the three...Ch. 8.6 - Prob. 7PCh. 8.6 - Prob. 8PCh. 8.8 - Prob. 9PCh. 8.9 - Prob. 10PCh. 8.9 - Prob. 12P
Ch. 8.9 - Prob. 13PCh. 8.10 - Prob. 14PCh. 8.10 - What orbitals contain the electrons represented as...Ch. 8.10 - Prob. 16PCh. 8.10 - Prob. 17PCh. 8.11 - Prob. 18PCh. 8.11 - Prob. 19PCh. 8.11 - Prob. 20PCh. 8.12 - Prob. 21PCh. 8.12 - Prob. 22PCh. 8.12 - Prob. 23PCh. 8.13 - Prob. 24PCh. 8.13 - Prob. 25PCh. 8.13 - Prob. 26PCh. 8.14 - Prob. 27PCh. 8.14 - Prob. 28PCh. 8.14 - Prob. 29PCh. 8.15 - Which member of each pair is the stronger acid?Ch. 8.15 - Which member of each pair is the stronger base? a....Ch. 8.15 - Rank the following compounds from strongest acid...Ch. 8.15 - Prob. 34PCh. 8.16 - Prob. 35PCh. 8.17 - Prob. 37PCh. 8.17 - Prob. 38PCh. 8.17 - Prob. 39PCh. 8.17 - Prob. 40PCh. 8.17 - Prob. 41PCh. 8.17 - Prob. 42PCh. 8.18 - Prob. 43PCh. 8.18 - Prob. 44PCh. 8.18 - Prob. 45PCh. 8.18 - Prob. 47PCh. 8.19 - Prob. 48PCh. 8.19 - Prob. 49PCh. 8.19 - Prob. 50PCh. 8.19 - Prob. 51PCh. 8.19 - Prob. 52PCh. 8.19 - Prob. 53PCh. 8.19 - Prob. 55PCh. 8.20 - Prob. 56PCh. 8 - Prob. 57PCh. 8 - Prob. 58PCh. 8 - Prob. 59PCh. 8 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Prob. 63PCh. 8 - Prob. 64PCh. 8 - Prob. 65PCh. 8 - Prob. 66PCh. 8 - Prob. 67PCh. 8 - Prob. 68PCh. 8 - Prob. 69PCh. 8 - Which compound is the strongest base?Ch. 8 - Prob. 71PCh. 8 - Prob. 72PCh. 8 - Prob. 73PCh. 8 - Prob. 74PCh. 8 - Prob. 75PCh. 8 - Prob. 76PCh. 8 - Prob. 77PCh. 8 - Prob. 78PCh. 8 - Purine is a heterocyclic compound with four...Ch. 8 - Prob. 80PCh. 8 - Why is the delocalization energy of pyrrole (21...Ch. 8 - Prob. 82PCh. 8 - Prob. 83PCh. 8 - Prob. 84PCh. 8 - A student obtained two products from the reaction...Ch. 8 - Prob. 86PCh. 8 - a. How could each of the following compounds be...Ch. 8 - Draw the products obtained from the reaction of...Ch. 8 - How would the following substituents affect the...Ch. 8 - Prob. 90PCh. 8 - The acid dissociation constant (Ka) for loss of a...Ch. 8 - Protonated cyclohexylamine has a Ka = 1 1011...Ch. 8 - Prob. 93PCh. 8 - Prob. 94PCh. 8 - Prob. 95PCh. 8 - Prob. 96PCh. 8 - Prob. 97PCh. 8 - a. Propose n mechanism for the following reaction:...Ch. 8 - Prob. 99PCh. 8 - As many as 18 different Diels-Alder products can...Ch. 8 - Prob. 101PCh. 8 - Prob. 102PCh. 8 - Prob. 103PCh. 8 - Prob. 104PCh. 8 - The experiment shown next and discussed in Section...Ch. 8 - Prob. 106PCh. 8 - Prob. 107PCh. 8 - Prob. 108PCh. 8 - Prob. 1PCh. 8 - Prob. 2PCh. 8 - Prob. 3PCh. 8 - Prob. 4PCh. 8 - Prob. 5PCh. 8 - Prob. 6PCh. 8 - Prob. 7PCh. 8 - Prob. 8PCh. 8 - Prob. 9PCh. 8 - Prob. 10PCh. 8 - Prob. 11PCh. 8 - Prob. 12P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY
How to Design a Total Synthesis; Author: Chemistry Unleashed;https://www.youtube.com/watch?v=9jRfAJJO7mM;License: Standard YouTube License, CC-BY