
EBK ORGANIC CHEMISTRY
6th Edition
ISBN: 9781260475685
Author: SMITH
Publisher: MCGRAW-HILL HIGHER EDUCATION
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 8.2, Problem 2P
Classify each
a.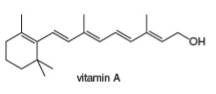 b.
b.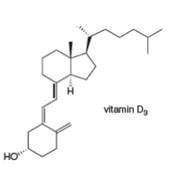
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Locate the isoprene units in each compound.
Which of the compounds can exist as enantiomers?
A. Isopropyl chloride
B. Bromochloromethane
C. Sec-butyl chloride
D. 1-chloro-2methylpentane
a. A and B
b. B and D
c. C and D
d. B and C
19. For the structure of "Uscharidin", a poisonous natural product, consider the following
statements and select the incorrect one.
a. The structure has two alcohol groups
b. The structure has two ketones
C. The structure has a trisubstituted double bond
d. The structure does not have a carboxylic acid
H.C
oh
and. The structure has an ester
OH
H,C
20. The relationship that exists for the following two pairs of stereoisomers is:
HC
H.C
CH
H,C OH
H,C. OH
H.
CH
H.
H.
couple A
couple
a. both pairs are constitutional isomers
b. couple A: diastereoisomers: couple B: constitutional isomers
C. couple A: enantiomers; couple B: constitutional isomers.
d. couple A: enantiomers; couple B: enantiomers
and. None of the above
Chapter 8 Solutions
EBK ORGANIC CHEMISTRY
Ch. 8.1 - Problem 8.1 Label the and carbons in each alkyl...Ch. 8.2 - Problem 8.2 Classify each alkene in the following...Ch. 8.2 - Prob. 3PCh. 8.2 - Prob. 4PCh. 8.2 - Problem 8.5 Label each pair of alkenes as...Ch. 8.2 - Problem 8.6 Which alkene in each pair is more...Ch. 8.2 - Problem 8.7 Several factors can affect alkene...Ch. 8.4 - Prob. 8PCh. 8.4 - Prob. 9PCh. 8.4 - Prob. 10P
Ch. 8.4 - Prob. 11PCh. 8.5 - Problem 8.12 What alkenes are formed from each...Ch. 8.6 - Prob. 13PCh. 8.6 - Problem 8.14 What alkenes are formed from each...Ch. 8.6 - Problem 8.15 How does each of the following...Ch. 8 - 8.24 Rank the alkenes shown in the ball-and-stick...Ch. 8 - Prob. 25PCh. 8 - 8.26 What is the major E2 elimination product...Ch. 8 - Prob. 27PCh. 8 - Prob. 28PCh. 8 - Prob. 29PCh. 8 - 8.30 Label each pair of alkenes as constitutional...Ch. 8 - Prob. 31PCh. 8 - Prob. 32PCh. 8 - Prob. 33PCh. 8 - For each of the following alkenes, draw the...Ch. 8 - Prob. 35PCh. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - What alkene is the major product formed from each...Ch. 8 - Prob. 39PCh. 8 - Prob. 41PCh. 8 - Draw the products formed when each dihalide is...Ch. 8 - Draw all of the substitution and elimination...Ch. 8 - Prob. 56PCh. 8 - 8.59 Draw a stepwise, detailed mechanism for each...Ch. 8 - Draw the major product formed when...Ch. 8 - Draw a stepwise, detailed mechanism for the...Ch. 8 - Explain why the reaction of with gives ...Ch. 8 - Draw a stepwise detailed mechanism that...Ch. 8 - Prob. 63PCh. 8 - 8.65 Explain the selectivity observed in the...Ch. 8 - Prob. 65PCh. 8 - Prob. 66PCh. 8 - 8.68 (a) Draw all products formed by treatment of...
Additional Science Textbook Solutions
Find more solutions based on key concepts
Determine the de Brogue wavelength of a. an electron moving at 1/10 the speed of light. b. a 400 g Frisbee movi...
Inorganic Chemistry
Q2. Which statement best defines chemistry?
a. The science that studies solvents, drugs, and insecticides
b. Th...
Introductory Chemistry (5th Edition) (Standalone Book)
For each of the following 2-dimensional shapes, determine the highest order rotation axis of symmetry.
Inorganic Chemistry
During the early part of the 20th century, sulfanilamide (an antibacterial drug) was only administered by injec...
Elementary Principles of Chemical Processes, Binder Ready Version
Determine [OH], [H+], and the pH of each of the following solutions. a. 1.0 M KCl b. 1.0 M KC2H3O2
Chemistry
Label each statement about the polynucleotide ATGGCG as true or false. The polynucleotide has six nucleotides. ...
General, Organic, & Biological Chemistry
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Identify the organic functional groups and reaction type for the following reaction.The reactant is a(n)a. ketopentoseb. aldopentosec. ketotriosed. alcohol pentosee. carboxylic acid tetroseThe product is a(n)a. aldohexosesb. alcohol pentosesc. carboxylic acid pentosesd. deoxypentosee. ketopentosesThe reaction type is:a. hemiacetal formationb. reduction (hydrogenation)c. hydrolysisd. acetal formatione. mutarotationf. oxidation (benedict's)arrow_forwardName some carcinogenic hydrocarbons.arrow_forwardSome alkenes have cis-trans isomers because a. the carbon atoms in the double bond are free to rotate. b. one of the carbon atoms in the double bond has two identical groups attached to it. c. all of the carbon atoms in the compound are rigid and cannot rotate. d. each of the carbon atoms in the double bond has four different groups attached to it. e. the carbon atoms in the double bond cannot rotate.arrow_forward
- . The following chemical reactants produce the ester ethylethanoate (C4H8O2):C2H6O + CH3COOHWhat type of reaction occurs to make ethyl ethanoate?a. condensationb. hydrolysisc. combustiond. acid-base reactionarrow_forwardFats belong to the class of organic compounds represented by the general formula, RCOOR', where R and R' represent hydrocarbon groups; therefore, fats are: a. ethers. b. soaps. c. esters. d. lipases.arrow_forwardClassify the following alcohols as primary, secondary, or tertiary: a. b. CH3CH2CH2CH2OH c.arrow_forward
- What type of hybridized orbital is present on carbon atoms bonded by a double bond? How many of these hybrid orbitals are on each carbon atom?arrow_forwardWhat type of hybridized orbital is present on carbon atoms bonded by a triple bond? How many of these hybrid orbitals are on each carbon atom?arrow_forwardAlcoholic beverages contain: a. wood alcohol. b. isopropyl alcohol. c. glyceryl alcohol. d. ethyl alcohol.arrow_forward
- Fats belong to the class of organic compounds represented by the general formula, RCOOR', where R and R' represent hydrocarbon groups. What is the name of the functional group present in fats? What functional group is common to all saponifiable lipids?arrow_forwardWhich of the following pairs represent structural isomers, and which are simply the same compound? a. and CH3CH2CH2CH3 b. and CH3CH2CH2CH2CH2CH3 c. and CH3CH2CH2CH3 d. andarrow_forward58. Which of the following statements incorrectly describes alcohols? A. The solubility of an alcohol is affected by the length of its carbon chain. B. The boiling point of an alcohol is affected by the length of its carbon chain. C. The solubility of an alcohol is affected by the shape of the molecule. D. Straight chain isomers will have a higher boiling point than the branched molecule. 59. Why do phenols have a higher boiling point than toluene despite having similar shape? A. The higher boiling point of phenol is attributed to the electronegativity of the oxygen. B. The higher boiling point of phenol is attributed to the hydrogen bonding. C. Both D. Neither 60. Which of the following statements incorrectly describes ethers? A. Ethers react easily with acids. B. Ethers are miscible with water. C. Ether serves as an electron acceptor group. D. None of the above Refer to the reaction to answer items 61 to 63. CH₂ OH KMnO4 H₂C 61. What type of reaction is catalyzed based on the…arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning
Chemistry for Today: General, Organic, and Bioche...ChemistryISBN:9781305960060Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. HansenPublisher:Cengage Learning General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning,
Organic And Biological ChemistryChemistryISBN:9781305081079Author:STOKER, H. Stephen (howard Stephen)Publisher:Cengage Learning, Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781337399425Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
 Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning
Introductory Chemistry: An Active Learning Approa...ChemistryISBN:9781305079250Author:Mark S. Cracolice, Ed PetersPublisher:Cengage Learning

Chemistry for Today: General, Organic, and Bioche...
Chemistry
ISBN:9781305960060
Author:Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning

Organic And Biological Chemistry
Chemistry
ISBN:9781305081079
Author:STOKER, H. Stephen (howard Stephen)
Publisher:Cengage Learning,

Introductory Chemistry: A Foundation
Chemistry
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning


Introductory Chemistry: An Active Learning Approa...
Chemistry
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Cengage Learning
07 Physical Properties of Organic Compounds; Author: Mindset;https://www.youtube.com/watch?v=UjlSgwq4w6U;License: Standard YouTube License, CC-BY