
Concept explainers
(a)
Interpretation: The conversion of compound 1 to compound 4 occurs with the formation of carbocation as an intermediate. The curved arrows for the given conversion of compound 1 to compound 4 is to be interpreted.
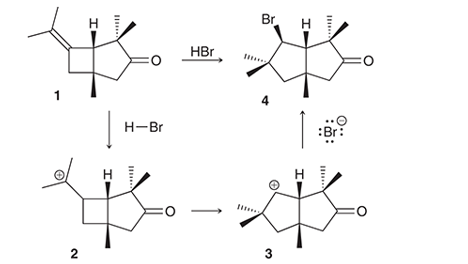
Concept introduction:
(b)
Interpretation: The conversion of compound 1 to compound 4 occurs with the formation of carbocation as an intermediate. The reason for the rearrangement of tertiary carbocation (structure 2) to secondary carbocation (structure 3) is to be interpreted.
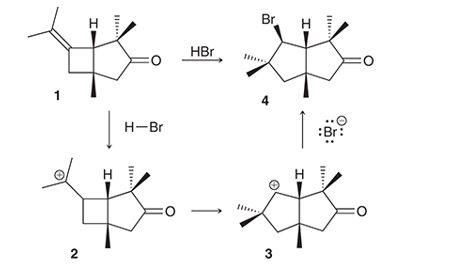
Concept introduction:
Alkenes are the unsaturated hydrocarbon that contains at least one double bond between the carbon atoms. The presence of pi bonds in these molecules makes them more reactive compared to saturated hydrocarbons; alkanes. The most common chemical reaction of alkenes is the addition reaction that mainly occurs with the formation of a carbocation.
Want to see the full answer?
Check out a sample textbook solution
Chapter 8 Solutions
EBK ORGANIC CHEMISTRY AS A SECOND LANGU
- prop 5. Draw each reaction entirely using line structures and follow the instructions below. Give the structures of compounds A through D in the following series of equations. NINH NH A C B + D Ba BID HBr. heat B D CH₂CH₂CH₂C=CC(CH₂),arrow_forwardQ6. Predict the structures of the final products of the following transformations. 1. NaNH, NH, CI 2, L, NH Q7. Identify the products of the following sequence of reactions. for Br KOH, CH₂CH₂OH BH₂/H₂O/H₂O₂, OHarrow_forward6) Consider the following reactions scheme below. NNH- H. Step II HO Step I Step II HC6 H HCN HO CEHSMGCI /H3O* a) Write a possible structure for compounds A and B. b) Determine reagents that needed and state the conditions (if any) for step I, step II and step III. c) Give name of the reaction for step I and step IlII.arrow_forward
- 2. Provide the mechanism for the reaction illustrated below. Show the relevant intermediate/transition state. Br N2OHarrow_forward6. Provide the reagents or the product for the following reactions. Br a b. HO он C. PB13arrow_forward3. Two cyclic products are possible from the Dieckmann condensation shown below, but only one product forms. 1. NaOEt, EtOH EtO OEt 2. H3O+ a. Provide the product of the Dieckmann condensation shown above. b. Provide a complete mechanism, using appropriate curved arrows to show the direction of electron flow, for the Dieckmann condensation. You must show the correct formal charges on atoms at each step. You must write out the full structure of each intermediate using an accepted chemical style. Clearly indicate which steps are reversible or unidirectional with the correct reaction arrows.arrow_forward
- 5. Provide a step-by-step mechanism to account for the product of each of the following reactions. Show the structure of each of the intermediates and use curved arrows to indicate electron flow in each of these steps. a) H-O. Na OMe (cat.) MeOHarrow_forward4. Use the reactant below to perform two separate reactions. Give the mechanism(s) and product(s) for each reaction. Show stereochemistry and be clear in your work. Use chair form. KOEt, EtOH t-Bu |||| H3O+arrow_forwardIn each box, provide either the missing reagents or the major organic product for each reaction below. Use wedge/dash notation to show stereochemistry (only if relevant). 1. NaH 2. CH3B1 NaOCH3 CH3OH OH PBR3 Но OH Br2 (excess) NaOH Br. OH NaH Brarrow_forward
- 4. Synthesis Using the carbon-containing starting material(s), propose a synthesis by drawing structures for all intermediates. The carbon atoms in the product must originate from the starting material(s) (or a carbene/carbenoid or CO2), but you may use as many equivalents of each starting material as you would like, and any reagent/reaction you know. (note: no mechanisms are required). OHarrow_forwardWhich reagent(s) IS NOT used in the synthesis problem shown below? H AH Note: This synthesis problem involves both alkene and alkyne reactions. If you're not sure how to start, make a list of objectives based on the changes you observe. Then consider the reagent options listed below, and use your notes to determine what each reagent does. O A. 1-iodoethane O B. NaH C. H2, Pb(OAc)2, Pd/CaCO3, quinoline O D. MCPBA O E. Na, NH3arrow_forward3. Substitution and Elimination. Consider the 2° alkyl halide shown below. Draw all major product(s) if it were to be treated with the following reagents. Be sure to consider stereochemistry and include structures of stereoisomers if present. H₂O Na OCH3. Acetonitrile CH3 ~Ire H3C- CH3 -OK* H3CS Na Acetonitrile Brarrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





