
ORGANIC CHEMISTRY TEXT PACKAGED - 2 YE
10th Edition
ISBN: 9781260024241
Author: Carey
Publisher: MCG/CREATE
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 8.6, Problem 8P
Instead of the three-step process of Mechanism 8.3, the following two-step mechanism might be considered:
1. 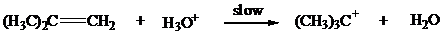
2. 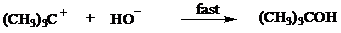
This mechanism cannot be correct! What is its fundamental flaw?
Mechanism 8.3
Acid-catalyzed Hydration of
The Overall Reaction
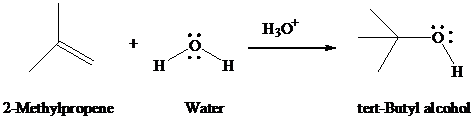
The Mechanism:
Step 1: Protonation of the carbon–carbon double bond in the direction that leads to the more stable carbocation:
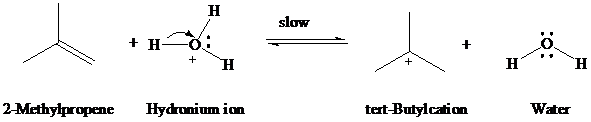
Step 2: Water acts as a nucleophile to capture
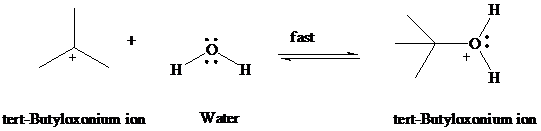
Step 3: Deprotonation of

Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
During the further development of other prostaglandin-like molecules, it is necessary to replace the cyclopentane ring with the cyclohexene as shown in the reaction scheme below. (Deuterium (D) is a heavier isotope of hydrogen)
a) Draw the most stable conformation of the starting material 8 and explain why this is the most stable conformation.
b) Draw the reaction mechanism for how 9 is formed from 8 and explain why 10 and 11 are not formed if one assumes the E2 mechanism.
Based on your answer to parts A - C, draw the correct regio- and stereoisomer of the product in the space provided.
Provide a systematic name of the substrate.
Provide a systematic name of the product (see section 8.3 for alkene nomenclature).
Draw a transition state structure of the reaction with partial bonds and partial charges indicated.
Draw an energy diagram for the E2 reaction on the previous page. Your reaction coordinate diagram should:
Include structures of starting materials, products, and transition states at correct relative energies.
Indicate the activation energy of the rate determining step and whether the reaction is endothermic or exothermic.
Explain the reaction mechanism of the reaction below? (Identify the type of reaction, the species (Nucleophile an electrophile) involve in the reaction after bond cleavage and the IUPAC name of the major product.
Chapter 8 Solutions
ORGANIC CHEMISTRY TEXT PACKAGED - 2 YE
Ch. 8.1 - What three alkenes yield 2-methylbutane on...Ch. 8.2 - Prob. 2PCh. 8.2 - Prob. 3PCh. 8.3 - Prob. 4PCh. 8.4 - Prob. 5PCh. 8.4 - Give a structural formula for the carbocation...Ch. 8.5 - Prob. 7PCh. 8.6 - Instead of the three-step process of Mechanism...Ch. 8.6 - The rates of hydration of the two alkenes shown...Ch. 8.6 - Is the electrophilic addition of hydrogen chloride...
Ch. 8.7 - You can calculate the equilibrium constant for the...Ch. 8.7 - Does the presence or absence of a catalyst such as...Ch. 8.7 - The gas phase reaction of ethanol with hydrogen...Ch. 8.8 - Prob. 14PCh. 8.8 - Hydroborationoxidation of -pinene, like its...Ch. 8.10 - Arrange the compounds 2-methyl-1-butene,...Ch. 8.10 - Give the structure of the product formed when each...Ch. 8.11 - Prob. 18PCh. 8.11 - Prob. 19PCh. 8.12 - Prob. 20PCh. 8.12 - Prob. 21PCh. 8.13 - Prob. 22PCh. 8.14 - Prob. 23PCh. 8.14 - Prob. 24PCh. 8 - How many alkenes yield...Ch. 8 - Prob. 26PCh. 8 - Catalytic hydrogenation of...Ch. 8 - Prob. 28PCh. 8 - Prob. 29PCh. 8 - Prob. 30PCh. 8 - Prob. 31PCh. 8 - A single epoxide was isolated in 7984% yield in...Ch. 8 - Prob. 33PCh. 8 - Prob. 34PCh. 8 - On catalytic hydrogenation over a rhodium...Ch. 8 - Prob. 36PCh. 8 - Prob. 37PCh. 8 - Prob. 38PCh. 8 - Prob. 39PCh. 8 - 1-Butene has a higher heat of hydrogenation than...Ch. 8 - Match the following alkenes with the appropriate...Ch. 8 - The heats of reaction were measured for addition...Ch. 8 - Complete the following table by adding + and -...Ch. 8 - Match the heats of hydrogenation (107 kJ/mol,...Ch. 8 - The iodination of ethylene at 25 C is...Ch. 8 - Specify reagents suitable for converting...Ch. 8 - (a) Which primary alcohol of molecular formula...Ch. 8 - Identify compounds A and B in the retrosynthesis...Ch. 8 - Identify compounds A and B in the retrosynthesis...Ch. 8 - Prob. 50PCh. 8 - On being heated with a solution of sodium ethoxide...Ch. 8 - Compound A (C7H15Br) is not a primary alkyl...Ch. 8 - Prob. 53PCh. 8 - Prob. 54PCh. 8 - A mixture of three alkenes (A, B, and C) was...Ch. 8 - Reaction of 3,3-dimethyl-1-butene with hydrogen...Ch. 8 - Dehydration of 2,2,3,4,4-pentamethyl-3-pentanol...Ch. 8 - Prob. 58PCh. 8 - East Indian sandalwood oil contains a hydrocarbon...Ch. 8 - Prob. 60PCh. 8 - Prob. 61PCh. 8 - Prob. 62PCh. 8 - Prob. 63PCh. 8 - Prob. 64PCh. 8 - On the basis of the mechanism of acid-catalyzed...Ch. 8 - As a method for the preparation of alkenes, a...Ch. 8 - Which of the following is the most reasonable...Ch. 8 - Prob. 68PCh. 8 - Oxymercuration Concerns about mercurys toxicity...Ch. 8 - Prob. 70DSPCh. 8 - Prob. 71DSPCh. 8 - Prob. 72DSPCh. 8 - Prob. 73DSPCh. 8 - Oxymercuration Concerns about mercurys toxicity...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Q3. 2-Bromopentane, when treated with alcoholic KOH yields a mixture of three alkenes A, B and C. Identify A, B and C. Which is predominant? Q4 Which statement below about Sn1 reactions is incorrect? (A) SN1 reactions are stepwise and have intermediates. (B) The slow step in a SN1 reaction is formation of the carbocation intermediate. (C) SN1 reactions have first order kinetics which means only the alkyl halide is involved in the rate limiting step. (D) The products of a SN1 reaction will be a pair of enantiomers. (E) An aprotic solvent is best for Sn1 reactions as they tend to help stabilize carbocation intermediates.arrow_forward3. Draw the systematic reaction mechanism for between the reaction of isoamyl alcohol and acetic acid. What are the products? What is the functional group of the major product? Does it have a scent? If so, what is the scent smells like?4. Charlie and Daniel wanted to make a drink containing 6.9% ethanol in the laboratory. However, there are three unlabelled reagent bottles that may contain these chemicals: ethanol, cyclohexane, and water. Devise a systematic flow chart to identify the ethanol and water in these three unlabelled reagent bottles.Assuming that the mixture in the reagent bottle containing ethanol are water and ethanol, what is the volume of the ethanol in the 132g of solution?5. Andrea and Yvette are going to cook the most delicious food that ever going to exist for their tired classmates that conducted the experiment in an organic chemistry laboratory. However, the stores are closed and they don’t have a table salt for the seasoning of their special dish so they need to…arrow_forward8.29 Give the mechanistic symbols (Sn1, Sn2, E1, E2) that are most consistent with each of the following statements: (g) Reactions proceeding by these mechanisms are stereospecific (h) These reaction mechanisms involve carbocation intermediates (i) These reaction mechanisms are the ones most likely to have been involved when the products are found to have a different carbon skeleton from the substratearrow_forward
- Below is a schematic representation of possible reaction that compound X can undergo.Use the scheme to answer the following questions. A.What is the IUPAC name for compound X B. What type of reactions is/are represented by (i) and(ii). C.Compound X undergo transitions through either (A) or (B) to produce compounds (1),(2),(3) and (4). Draw the structure of (A) and (B).arrow_forwardorganic chemistry 7) What is the final product expected from the following reaction?arrow_forward1(a) Explain briefly what is meant by ONE of the following terms (use words and diagrams).(i) Markovnikov’s rule(ii) Transition state 1(b) Draw the mechanism (using structures, arrows and appropriate symbols) for the formation of thefollowing products:arrow_forward
- Use the roadmap reactions to determine the steps reactions and the intermediate products for each synthesis. Help with 3&4 only!arrow_forward(19, 9) Draw a structural formula for the major organic product of the reactions shown below. You do not have to consider stereochemistry.arrow_forward10 What is the rate of reaction when the transition state free energy is 22.84 kJ/mol? What is the rate of reaction when the transition state free energy is 142 kJ/mol? SHOW YOUR WORK.arrow_forward
- Consider the following SN2 reaction.a.Draw a mechanism using curved arrows. b. Draw an energy diagram. Label the axes, the reactants, products, Ea, and ΔH°. Assume that the reaction is exothermic. c. Draw the structure of the transition state. d.What is the rate equation? e.What happens to the reaction rate in each of the following instances? [1] The leaving group is changed from Br− to I−; [2] The solvent is changed from acetone to CH3CH2OH; [3] The alkyl halide is changed from CH3(CH2)4Br to CH3CH2CH2CH(Br)CH3; [4] The concentration of −CN is increased by a factor of five; and [5] The concentrations of both the alkyl halide and −CN are increased by a factor of five.arrow_forward5..8. What is the expected major for the following reaction ?arrow_forward1. For the following reaction, what is the rate law ? (image) 2. is the following nucleophile strong or weak HO^- a) strong b) weak c) not a nucleophile 3) what set of reaction conditions should favor an SN2 reaction on 2-bromo-3-methylbutane a) weak nucleophile in a protic solvent b) weal nucleophile in aprotic solvent c) strong nucleophile in a protic solvent d) strong nucleophile in a aprotic solventarrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY