
Concept explainers
A piston–cylinder device initially contains 1.4 kg of refrigerant-134a at 100 kPa and 20°C. Heat is now transferred to the refrigerant from a source at 150°C, and the piston, which is resting on a set of stops, starts moving when the pressure inside reaches 120 kPa. Heat transfer continues until the temperature reaches 80°C. Assuming the surroundings to be at 25°C and 100 kPa, determine (a) the work done, (b) the heat transfer, (c) the exergy destroyed, and (d) the second-law efficiency of this process.
FIGURE P8–48
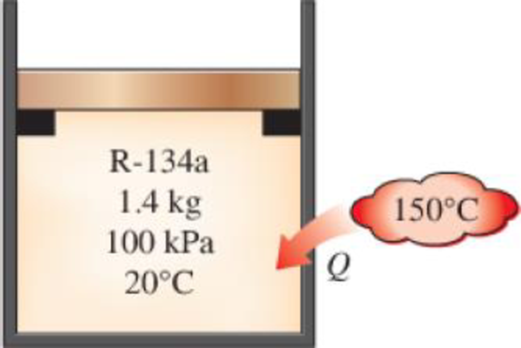
(a)
The work done.
Answer to Problem 48P
The work done is
Explanation of Solution
Express the boundary work done.
Here, mass is
Conclusion:
Perform the unit conversion of initial pressure and final pressure from
Refer Table A-13, “superheated refrigerant-134a”, and write the properties corresponding to initial pressure of
Here, initial specific volume, internal energy and entropy is
Refer Table A-13, “superheated refrigerant-134a”, and write the properties corresponding to final pressure of
Write the formula of interpolation method of two variables.
Here, the variables denote by x and y is final pressure and final specific volume respectively.
Show the final specific volume at
|
Final pressure |
Final specific volume |
| 0.10 | 0.28465 |
| 0.12 | |
| 0.14 | 0.20242 |
Substitute
Show the final specific internal energy at
|
Final pressure |
Final specific internal energy |
| 0.10 | 297.10 |
| 0.12 | |
| 0.14 | 296.77 |
Substitute
Show the final specific entropy at
|
Final pressure |
Final specific entropy |
| 0.10 | 1.2573 |
| 0.12 | |
| 0.14 | 1.2289 |
Substitute
Thus, write the values obtained from interpolation method:
Substitute
Hence, the work done is
(b)
The heat transfer.
Answer to Problem 48P
The heat transfer is
Explanation of Solution
Express heat transfer.
Conclusion:
Substitute 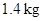 for
for  ,
,
(c)
The exergy destroyed.
Answer to Problem 48P
The exergy destroyed is
Explanation of Solution
Express the exergy destruction.
Here, entropy generation is
Express the entropy generation by taking entropy balance on an extended system.
Here, net entropy transfer by heat and mass is
Conclusion:
Substitute
Substitute
Hence, the exergy destroyed is
(d)
The second law efficiency of the process.
Answer to Problem 48P
The second law efficiency of the process is
Explanation of Solution
Express exergy expended.
Here, efficiency for reversible cycle is
Express the second law efficiency.
Conclusion:
Substitute
Substitute
Hence, the second law efficiency of the process is
Want to see more full solutions like this?
Chapter 8 Solutions
THERMODYNAMICS (LL)-W/ACCESS >IP<
- Refrigerant-134a enters an adiabatic compressor as saturated vapor at 30 psia at a rate of 20 ft3 /min and exits at 70 psia pressure. If the isentropic efficiency of the compressor is 80 percent, determine the second-law efficiency of the compressor. Assume the surroundings to be at 75°F.arrow_forwardA 0.06-m3 rigid tank initially contains refrigerant- 134a at 0.8 MPa and 100 percent quality. The tank is connected by a valve to a supply line that carries refrigerant- 134a at 1.2 MPa and 36°C. Now the valve is opened, and the refrigerant is allowed to enter the tank. The valve is closed when it is observed that the tank contains saturated liquid at 1.2 MPa. Determine (a) the mass of the refrigerant that has entered the tank and (b) the amount of heat transfer.arrow_forwardSteam enters a turbine steadily at a flow rate of 1 kg/s at 7 MPa and 500 degrees and exits as saturated steam at 40 kPa. If there is a heat loss of 10 kW from the turbine, what will be the power produced by the turbine?arrow_forward
- What is the minimum internal energy that steam can achieve as it is expanded adiabatically in a closed system from 1500 kPa and 320°C to 100 kPa?arrow_forwardHow does useful work differ from actual work? For what kinds of systems are these two identical?arrow_forwardCan a steady-flow system involve boundary work?arrow_forward
- Saturated refrigerant-134a vapor at 15 psia is compressed reversibly in an adiabatic compressor to 80 psia. Determine the work input to the compressor. What would your answer be if the refrigerant were first condensed at constant pressure before it was compressed?arrow_forwardAir enters the compressor of a gas-turbine plant at ambient conditions of 100 kPa and 30°C with a low velocity and exits at 1 MPa and 367 °C with a velocity of 6×103 m/min. The compressor is cooled at a rate of 1800 kJ/min, and the power input to the compressor is 250 kW. Determine the mass flow rate of air through the compressor.arrow_forwardSteam enters an adiabatic turbine at 8 MPa and 500°C at a rate of 2.7 kg/s and leaves at 20 kPa. If the power output of the turbine is 2.5 MW, determine the temperature of the steam at the turbine exit. Neglect kinetic energy changesarrow_forward
- Steam enters an adiabatic turbine at 800 psia and 900F and leaves at a pressure of 40 psia. Determine the maximum amount of work that can be delivered by this turbine.arrow_forwardA piston–cylinder device initially contains 1.4 kg of refrigerant-134a at 100 kPa and 20°C. Heat is now transferred to the refrigerant from a source at 150°C, and the piston, which is resting on a set of stops, starts moving when the pressure inside reaches 120 kPa. Heat transfer continues until the temperature reaches 80°C. Assuming the surroundings to be at 25°C and 100 kPa, determine the heat transfer.arrow_forwardNitrogen gas is compressed from 80 kPa and 27°C to 480 kPa by a 10-kW compressor. Determine the mass flow rate of nitrogen through the compressor, assuming the compression process to be isothermal.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





