
a)
The quality or temperature (if superheated) of the steam at the turbine exit.
a)
Explanation of Solution
Given:
Pressure of steam at state 3
Pressure of steam at state 1
Temperature of steam at the state 3
Net power output of the cycle
Isentropic efficiency of the boiler
Isentropic efficiency of the pump
Calculation:
Draw the
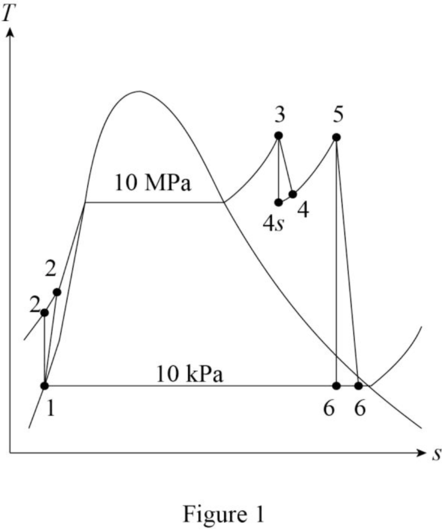
The entropies are constant for the process 3 to 4s and process 5 to 6s.
Refer Table A-5, “Saturated water-Pressure table”, obtain the specific enthalpy and specific volume at state 1 corresponding to the pressure of
Calculate the work done by the pump during process 1-2
Calculate the specific enthalpy at state 2
Refer Table A-6, “Superheated water”, obtain the specific enthalpy and specific entropy at state 3 corresponding to the pressure of
Refer Table A-5, “Saturated water-Pressure table”, obtain the following properties corresponding to the pressure of
Calculate the specific enthalpy at state 4
Refer Table A-6, “Superheated water”, obtain the specific enthalpy and specific entropy at state 5 corresponding to the pressure of
Refer Table A-5, “Saturated water-Pressure table”, obtain the following properties corresponding to the pressure of
Calculate the quality at state 6s
Calculate the specific enthalpy at state 6s
Since the specific enthalpy at state 6s is greater than
Refer Table A-5, “Saturated water-Pressure table”, obtain the temperature of the steam corresponding to the pressure of
Thus, the temperature of the steam at the turbine exit is
b)
The thermal efficiency of the cycle.
b)
Explanation of Solution
Calculation:
Calculate the thermal efficiency of the cycle
Thus, the thermal efficiency of the cycle is
c)
The mass flow rate of the steam.
c)
Explanation of Solution
Calculation:
Calculate the mass flow rate of the steam
Thus, the mass flow rate of the steam is
Want to see more full solutions like this?
Chapter 9 Solutions
Loose Leaf For Fundamentals Of Thermal-fluid Sciences Format: Looseleaf
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





