
Compare the thermal efficiency of the cycle when it is operated such that the saturated liquid enters the pump against that when the sub-cooled liquid enters the pump.
Explanation of Solution
Given:
Pressure of steam at the boiler
Pressure of steam at the condenser
Temperature of steam at the turbine inlet
Temperature of sub-cooled liquid
Calculation:
Draw the
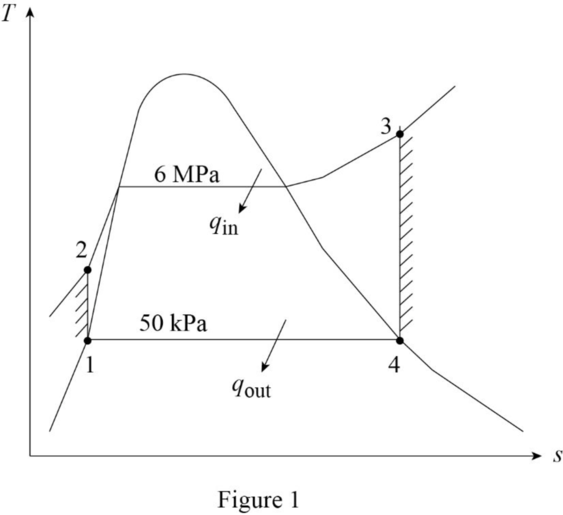
The pressures are constant for the process 2 to 3 and process 4 to 1.
The entropies are constant for the process 1 to 2 and process 3 to 4.
The thermal efficiency of the cycle when it is operated such that when the saturated liquid enters the pump:
Refer Table A-5, “Saturated water-Pressure table”, obtain the specific enthalpy and specific volume at state 1 corresponding to the pressure of
Calculate the work done by the pump during process 1-2
Calculate the specific enthalpy at state 2
Refer Table A-6, “Superheated water”, obtain the specific enthalpy and temperature at state 3 corresponding to the pressure of
Refer Table A-5, “Saturated water-Pressure table”, obtain the following properties corresponding to the pressure of
Calculate the quality of steam at state 4
Calculate the specific enthalpy at state 4
Calculate the thermal efficiency of the cycle
The thermal efficiency of the cycle when it is operated such that when the sub-cooled liquid enters the pump:
Refer Table A-5, “Saturated water-Pressure table”, obtain the saturation temperature corresponding to the pressure of
Calculate the temperature at state 1
Refer Table A-4, “Saturated water-Temperature table”, obtain the specific enthalpy and specific volume at state 1 corresponding to the temperature of
Calculate the work done by the pump during process 1-2
Calculate the enthalpy at state 2
Calculate the thermal efficiency of the cycle
By comparing the thermal efficiencies of the cycle, it is found that the thermal efficiency slightly reduces because of the sub-cooling at the inlet of the pump.
Want to see more full solutions like this?
Chapter 9 Solutions
FUND.OF THRMAL FLD. SCI. W/ACC. CODE>C
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





