
Concept explainers
(a)
Interpretation:
The
Concept Introduction:
Functional group: They are certain substitutes in the organic molecules which determine the characteristic reactions taking place in it.
There are different types of functional groups and it includes
The cyclic group of atoms with formula
(a)
Explanation of Solution
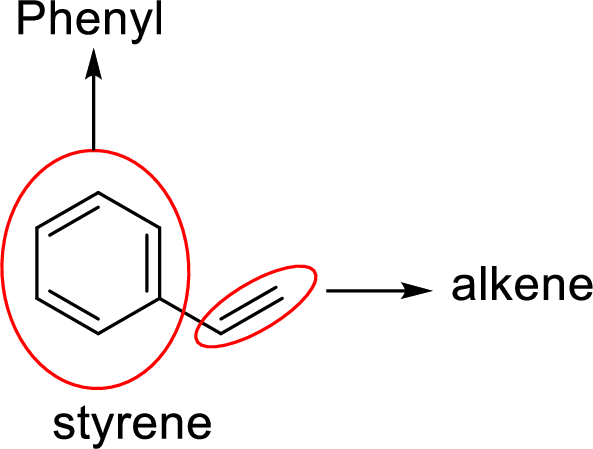
Analyzing the given structure for given styrene compound clearly shows that it contains one phenyl group since one
(b)
Interpretation:
The functional groups present in ethylene glycol have to be identified.
Concept Introduction:
Functional group: They are certain substitutes in the organic molecules which determine the characteristic reactions taking place in it.
There are different types of functional groups and it includes alkane, alcohol, aldehyde, amine, ether, carboxylic acid etc.
The entity formed as a result of bond formation between oxygen and hydrogen is called hydroxyl group. This is represented by
(b)
Explanation of Solution
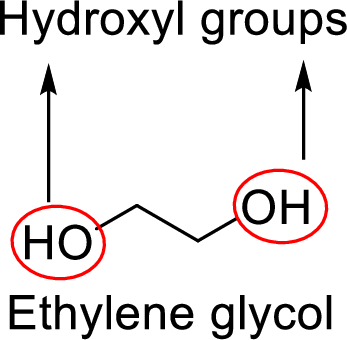
Analyzing the structure for given ethylene compound clearly shows that it contains two hydroxyl groups since hydroxyl group is entity formed by bond formation between oxygen and hydrogen atoms.
(c)
Interpretation:
The functional groups present in terephthalic acid have to be identified.
Concept Introduction:
Functional group: They are certain substitutes in the organic molecules which determine the characteristic reactions taking place in it.
There are different types of functional groups and it includes alkane, alcohol, aldehyde, amine, ether, carboxylic acid etc.
The cyclic group of atoms with formula
Alkenes: Alkenes are one of the important types of hydrocarbon which have at least one carbon-carbon double bond (
The functional group carboxylic acid is one which contains one hydroxyl group gets bonded with one carbonyl group that is one
The entity formed as a result of bond formation between oxygen and hydrogen is called hydroxyl group. This is represented by
(c)
Explanation of Solution
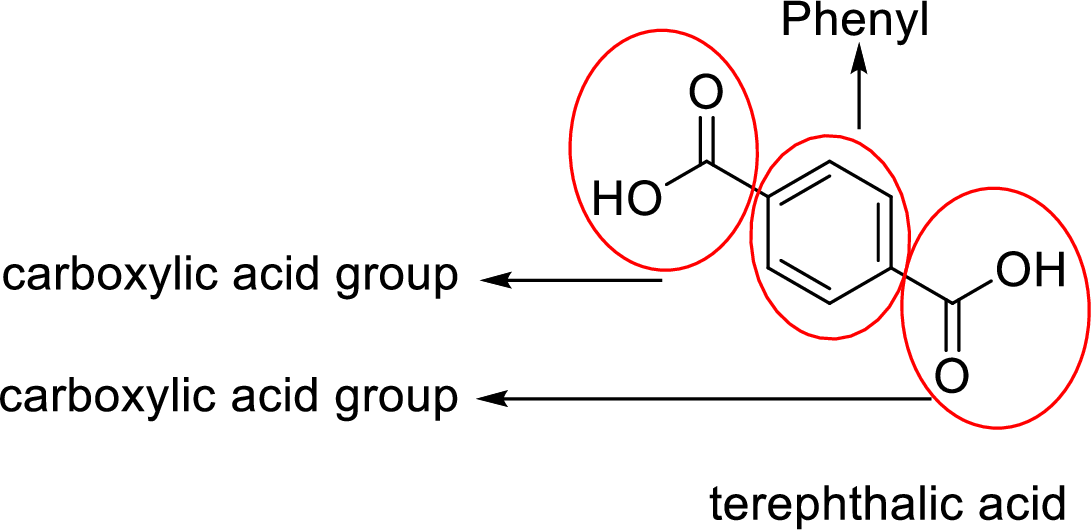
Analyzing the given structure for given terephthalic acid compound clearly shows that it contains one phenyl group since one
(d)
Interpretation:
The functional groups present in the amino acid in which
Concept Introduction:
Functional group: They are certain substitutes in the organic molecules which determine the characteristic reactions taking place in it.
There are different types of functional groups and it includes alkane, alcohol, aldehyde, amine, ether, carboxylic acid etc.
The functional group carboxylic acid is one which contains one hydroxyl group gets bonded with one carbonyl group that is one
The entity formed as a result of bond formation between oxygen and hydrogen is called hydroxyl group. This is represented by
Carboxylic acid: One
Amines are the derivatives of ammonia
(d)
Explanation of Solution
Amino acid had both amino functional group and carboxyl functional group in a molecule.
Amino functional group is
(e)
Interpretation:
The functional groups present in hexamethylenediamine have to be identified.
Concept Introduction:
Functional group: They are certain substitutes in the organic molecules which determine the characteristic reactions taking place in it.
There are different types of functional groups and it includes alkane, alcohol, aldehyde, amine, ether, carboxylic acid etc.
Amines are the derivatives of ammonia
Depending on the number of carbon side chain of the nitrogen, different types of amines can form.
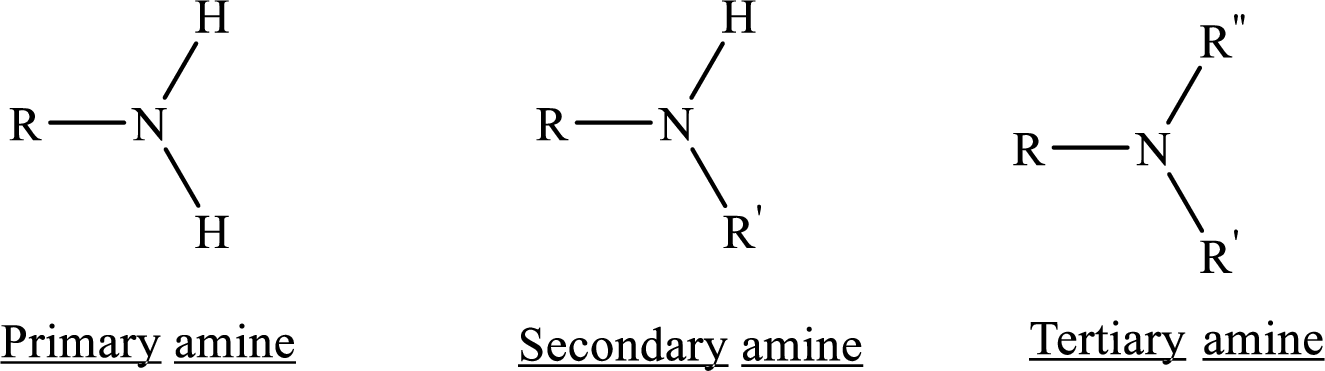
(e)
Explanation of Solution
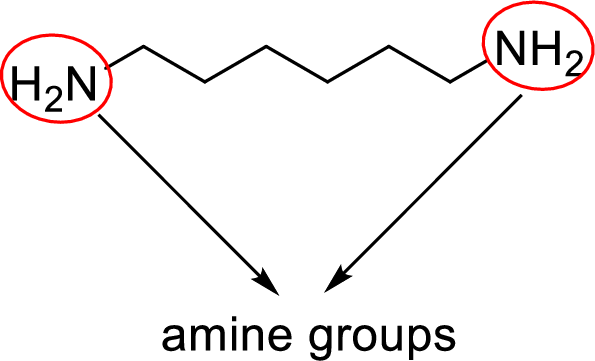
Analyzing the given structure for given hexamethylenediamine compound clearly shows that it contains two
(f)
Interpretation:
The functional groups present in adipic acid have to be identified.
Concept Introduction:
Functional group: They are certain substitutes in the organic molecules which determine the characteristic reactions taking place in it.
There are different types of functional groups and it includes alkane, alcohol, aldehyde, amine, ether, carboxylic acid etc.
Carboxylic acid: One
The functional group carboxylic acid is one which contains one hydroxyl group gets bonded with one carbonyl group that is one
The entity formed as a result of bond formation between oxygen and hydrogen is called hydroxyl group. This is represented by
(f)
Explanation of Solution
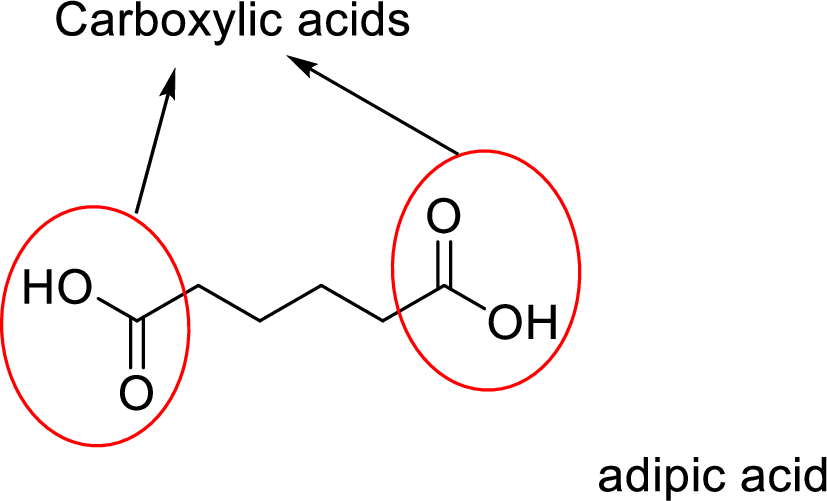
Analyzing the structure for given adipic acid compound clearly shows that it contains two carboxylic acids since carboxylic acid entity is one which contains one hydroxyl group gets bonded with carbonyl group.
Want to see more full solutions like this?
Chapter 9 Solutions
Laboratory Manual Chemistry in Context
 Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
Introductory Chemistry: A FoundationChemistryISBN:9781285199030Author:Steven S. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning
World of Chemistry, 3rd editionChemistryISBN:9781133109655Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCostePublisher:Brooks / Cole / Cengage Learning Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning- Chemistry: Matter and ChangeChemistryISBN:9780078746376Author:Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl WistromPublisher:Glencoe/McGraw-Hill School Pub Co
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div
World of ChemistryChemistryISBN:9780618562763Author:Steven S. ZumdahlPublisher:Houghton Mifflin College Div





