
Write the structure of the major organic product isolated from the reaction of
Hydrogen (
Hydrogen (
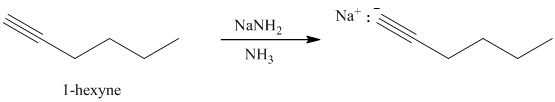
Sodium amide in liquid ammonia
Product in part (c) treated with 1-bromobutane
Product in part (c) treated with tert-butyl bromide
Hydrogen chloride (
Hydrogen chloride (
Chlorine (
Chlorine (
Aqueous sulfuric acid, mercury
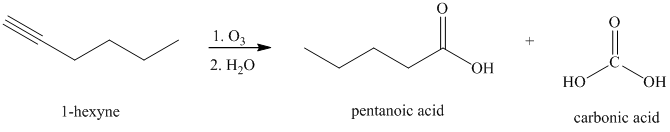
Ozone followed by hydrolysis
Interpretation:
The structure of the major product isolated from the reaction of
Concept introduction:
In the presence a metal catalyst such as platinum, palladium, nickel, or rhodium, two molar equivalents of hydrogen get added across the triple bond of an alkyne to yield an alkane. In hydrogenation reaction, two hydrogen atoms get added to each carbon atom across the triple bond.
Hydrogenation reaction of alkynes to alkenes using the Lindlar catalyst is stereoselective. In this reaction only the cis alkene is produced.
Terminal alkynes react with
Addition of hydrogen halides to alkynes gives alkenyl halides. The regioselectivity of this reaction follows Markovnikov’s rule. The hydrogen atom from hydrogen halide adds to the carbon in the alkyne that has the more number of hydrogens, and the halide adds to the carbon with the lower number of hydrogens.
In presence of excess of hydrogen halide, the sequential addition of two molecules of hydrogen halide to the carbon-carbon triple bond yields geminal dihalides.
The reaction of chlorine and bromine with alkynes yields tetrahaloderivatives. In this reaction, two molecules of the halogen get added to the triple bond. During the reaction, a dihaloalkene is get formed. When alkyne and the halogen are present in equimolar quantity, the dihaloalkene intermediate get formed and isolated. The stereochemistry of addition reaction is anti.
Alkynes, when subjected to ozonolysis followed by hydrolysis, produce carboxylic acids.
Answer to Problem 24P
Solution:
a)
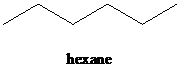
b)
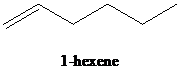
c)
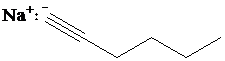
d)
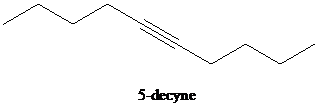
e)
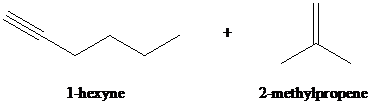
f)
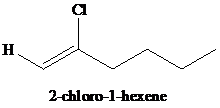
g)
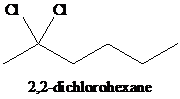
h)

i)
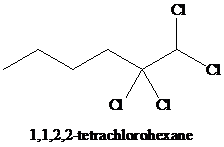
j)
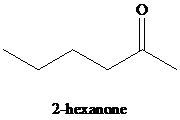
k)
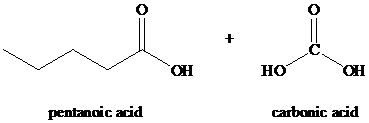
Explanation of Solution
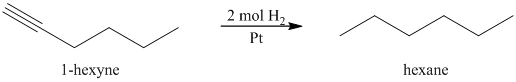
a) The reaction of
In the presence a metal catalyst such as platinum, palladium, nickel, or rhodium, two molar equivalents of hydrogen get added across the triple bond of an alkyne to yield an alkane. In case of
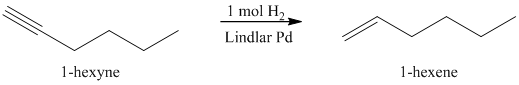
b) The reaction of
Reduction over Lindlar palladium catalyst with one mole of hydrogen partially reduces an alkyne to the corresponding cis alkene.
c) The reaction of
Terminal alkynes react with
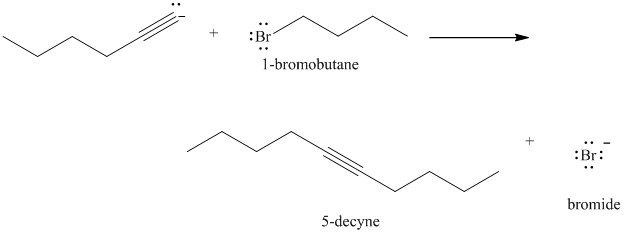
d) The reaction of
In the reaction of alkyl halide and sodium acetylide, the acetylide ion acts as a nucleophile. It displaces halide from carbon and forming a new carbon-carbon bond. This reaction follows
The product formed in part (c) is the anion formed by
The product formed is
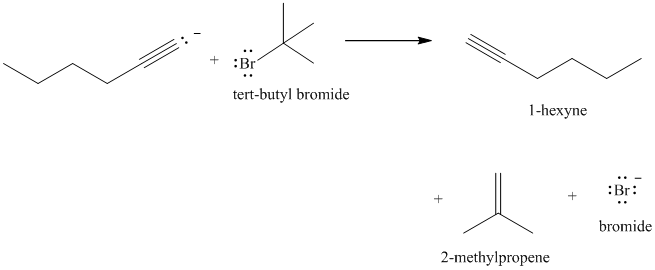
e) The reaction of
Acetylide anions are much more basic than hydroxide. Acetylide anions react with secondary and tertiary alkyl halides by elimination. Tert-butyl bromide is a tertiary alkyl halide and does not react by the
The product formed in part (c) is the anion formed by
f) The reaction of
Hydrogen halides add to alkynes to form alkenyl halides. When
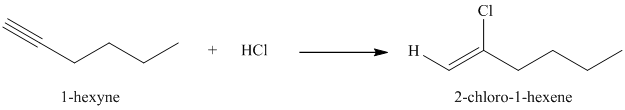
g) The reaction of
In presence of excess of hydrogen halide, the sequential addition of two molecules of hydrogen halide to the carbon-carbon triple bond yields geminal dihalides. The second mole addition of hydrogen halide to the initially formed alkenyl halide is done in accordance with Markovnikov’s rule. Overall, both protons get attached to the same carbon and both halogens to the adjacent carbon atom. In
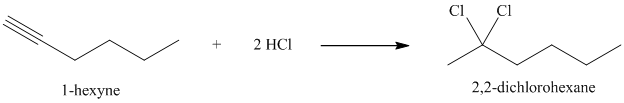
h) The reaction of
When one mole of halogen molecule is added to an alkyne, the product formed is a dihaloalkene. One halogen atom gets attached to each of the triple bonded carbon atoms, and it is reduced to a double bond.
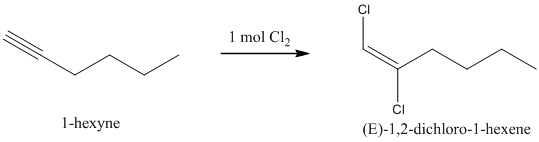
i) The reaction of
Alkynes react with two moles of chlorine to yield tetrachloroalkanes. Two molecules of the chlorine add to the triple bond. The stereochemistry of addition is anti.
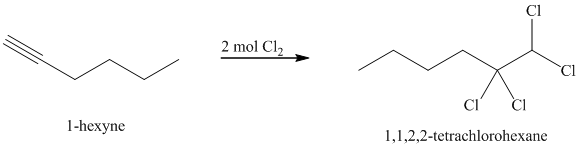
j) The reaction of
Hydration of alkynes employs aqueous sulfuric acid as the reaction medium and mercury(II) sulfate as a catalyst. The product formed is an enol which tautomerizes to the corresponding ketone. Markovnikov’s rule is followed in hydration of alkynes. Terminal alkynes yield methyl substituted ketones.
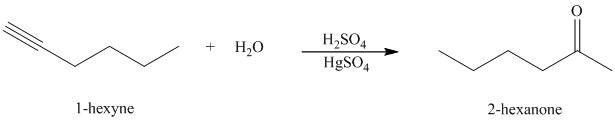
k) The reaction of
Alkynes, when subjected to ozonolysis followed by hydrolysis, produce carboxylic acids. If carbonic acid is one of the products, it dissociates into carbon dioxide and water.
Want to see more full solutions like this?
Chapter 9 Solutions
ORGANIC CHEMISTRY-PACKAGE >CUSTOM<
- A hydrocarbon (X), with the molecular formula: C8H14 is reduced in presence of sodium and liquid ammonia to give the only product (Y) with the molecular formula: C8H16. Compounds X and Y both resulting 2,5-dimethylhexane when treated with hydrogen and platinum catalyst (H2/Pt). As a result of the oxidative cleavage of compound Y (by using KMnO4 / H2SO4), a single carboxylic acid derivative with C4H8O2 molecular formula is formed. Again, as a result of the reaction of Y with perbenzoic acid, the chiral compound C8H14O is observed, but the reaction of compound Y with bromine gives the achiral C8H14Br2 as the product.arrow_forwardWrite the structure of the major organic product formed in the reaction of 1-pentene with each of the following: (a) Hydrogen chloride (b) Dilute sulfuric acid (c) Diborane in diglyme, followed by basic hydrogen peroxide (d) Bromine in carbon tetrachloride (e) Bromine in water (f) Peroxyacetic acid (g) Ozone (h) Product of part (g) treated with zinc and water (i) Product of part (g) treated with dimethyl sulfide (CH3)2Sarrow_forwardAn unknown compound A of molecular formula C10H18O reacts with H2SO4 to form two compounds (B and C)of molecular formula C10H16. B and C both react with H2 in the presence of Pd-C to form decalin. Ozonolysis of B forms D, and ozonolysis of C forms a diketone E of molecular formula C10H16O2. Identify the structures of compounds A, B, C, and E.arrow_forward
- Draw the structure of a compound of molecular formula C6H10 that reacts with H2 in the presence of Pd-C but does not react with H2 in the presence of Lindlar catalyst.arrow_forwardCompound A is first reacted with methylamine in the presence of acid and then treated with NaBH3CN. Using the spectroscopic data given, what is the structure of the product after step 1?arrow_forwardProvide the structure of the major organic product of the following reaction and? explain the stereochemistry which results in this product. 2-Pentanol reacting with 1.) PBr3, pyridine 2.) NaCNarrow_forward
- Compound A of molecular formula C8H14 is reduced by sodium in liquid ammonia to give compound B of molecular formula C8H16. product (Y).Both A and B undergo hydrogenation in the presence of a platinum catalyst to give 2,5-dimethylhexane. Ozonolysis of B with an oxidative workup produces a carboxylic acid of molecular formula C4H8O2. Reaction of B with a peroxyacid gives a chiral C8H14O product, but reaction with bromine gives an achiral C8H14Br2 product. What are the identities of A and B?arrow_forwardShow how you can synthesize the following compounds starting with benzene, toluene, and alcohols containing no morethan four carbon atoms as your organic starting materials. Assume that para is the major product (and separable fromortho) in ortho, para mixtures.(a) pentan-1-amine (b) N-methylbutan-1-aminearrow_forward1) How will you describe whether any compound has been oxidized or reduced? Support the answer with suitable examples. 2)Why carboxylic acid with a carbonyl group at 3rd position can be decarboxylated? 3) Explain why electrophilic aromatic substitution in Pyrrole takes place at C-2 positions whereas, in Pyridine it takes place at C-3 position? 4) List the following esters in order of decreasing reactivities towards hydrolysis with reason: Methyl benzoate, p-cyano methyl benzoate and p-hydroxy methyl benzoate 5)LDA is the base of choice for carbonyl compound to completely convert into enolate. Why?arrow_forward
- Provide the sequence to complete the synthesis of cyclohexanol to cis-1,2-cyclohexandiol.arrow_forwardStarting with the following compounds, outline a practical synthesis of 1-butanolarrow_forwardCompound A produce compound D while undergo Friedel Crafts Alkylation. Compound D is then oxidized and produce compound E (C11H12O3) as a major product.What are the possible structural formula of compound D and E?arrow_forward
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





