
Loose Leaf Student Solutions Manual Organic Chemistry
10th Edition
ISBN: 9781259968907
Author: Francis Carey, Robert Giuliano
Publisher: McGraw-Hill Education (edition 10)
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 37P
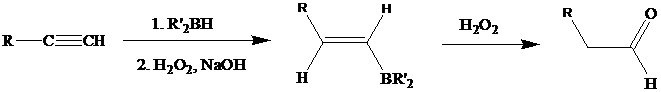
The oxidation step involves an enol intermediate. Using Mechanism
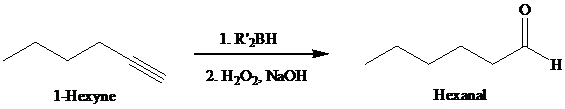
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Alkylation of benzene with 1-chlorobutane in the presence of AlCl3 gave not only the expected butylbenzene product but also, as a major product, (1-methylpropyl)benzene.
Write an equation for the reaction
Propose a mechanism to account for the formation of butylbenzene
Propose a mechanism to account for the formation of (1-methylpropyl)benzene
When 1 mole of buta-1,3-diene reacts with 1 mole of HBr, both 3-bromobut-1-ene and 1-bromobut-2-ene are formed. Propose a mechanism to account for this mixture of products.
Draw structural formulas for the isomeric carbocation intermediates formed on treatment of each alkene with HCl. Label each carbocation 1°, 2°, or 3° and state which of the isomeric carbocations forms more readily.
Chapter 9 Solutions
Loose Leaf Student Solutions Manual Organic Chemistry
Ch. 9.1 - Prob. 1PCh. 9.2 - Prob. 2PCh. 9.4 - How do bond distances and bond strengths change...Ch. 9.5 - Complete each of the following equations to show...Ch. 9.6 - Prob. 5PCh. 9.6 - Which of the alkynes of molecular formula C5H8 can...Ch. 9.7 - Give the structures of three isomeric dibromides...Ch. 9.7 - Prob. 8PCh. 9.9 - Write a series of equations showing how you could...Ch. 9.9 - Write a series of equations showing how to prepare...
Ch. 9.10 - Prob. 11PCh. 9.11 - Give the structure of the enol formed by hydration...Ch. 9.11 - Prob. 13PCh. 9.13 - Prob. 14PCh. 9.14 - Prob. 15PCh. 9 - Provide the IUPAC name for each of the following...Ch. 9 - Prob. 17PCh. 9 - All compounds in Problem 9.17 are isomers except...Ch. 9 - Prob. 19PCh. 9 - Write structural formulas for all the alkynes of...Ch. 9 - Prob. 21PCh. 9 - Prob. 22PCh. 9 - The alkane formed by hydrogenation of...Ch. 9 - Write the structure of the major organic product...Ch. 9 - Write the structure of the major organic product...Ch. 9 - When 2-heptyne was treated with aqueous sulfuric...Ch. 9 - Prob. 27PCh. 9 - Prob. 28PCh. 9 - Prob. 29PCh. 9 - Show by writing appropriate chemical equations how...Ch. 9 - Show by writing appropriate chemical equations how...Ch. 9 - Diphenylacetylene can be synthesized by the double...Ch. 9 - (Z)-9-tricosene [ (Z)-CH3(CH2)7CH=CH(CH2)12CH3 ]...Ch. 9 - Prob. 34PCh. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Alkynes undergo hydroboration to give...Ch. 9 - Prob. 38DSPCh. 9 - Prob. 39DSPCh. 9 - Prob. 40DSPCh. 9 - Prob. 41DSPCh. 9 - Thinking Mechanistically About Alkynes The...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- When warmed in dilute sulfuric acid, 1-phenyl-1,2-propanediol undergoes dehydration and rearrangement to give 2-phenylpropanal. (a) Propose a mechanism for this example of a pinacol rearrangement (Section 10.7). (b) Account for the fact that 2-phenylpropanal is formed rather than its constitutional isomer, 1-phenyl-1-propanone.arrow_forwardWhen cis-4-chlorocyclohexanol is treated with sodium hydroxide in ethanol, it gives mainly the substitution product trans-1,4-cyclohexanediol (1). Under the same reaction conditions, trans-4-chlorocyclohexanol gives 3-cyclohexenol (2) and the bicyclic ether (3). (a) Propose a mechanism for formation of product (1), and account for its configuration. (b) Propose a mechanism for formation of product (2). (c) Account for the fact that the bicyclic ether (3) is formed from the trans isomer but not from the cis isomer.arrow_forwardA reaction flask contains 2-bromopentane in an ethanolic solution of sodium ethoxide at room temperature and result in the formation of two olefnic products. What is responsible for the formation of major and minor products. A. Different activated complex involved in the mechanism. B. Bimolecular nucleophilic substitution reaction. C. Bimolecular elimination reaction D. The presence of sodium ethoxide. E. The hybridization nature of secondary carbocationarrow_forward
- Diphenylacetylene can be synthesized by the double dehydrohalogenation of 1,2-dibromo-1,2-diphenylethene. The sequence starting from (E)-1,2-diphenylethene consists of bromination to give the dibromide, followed by dehydrohalogenation to give a vinylic bromide, then a second dehydrohalogenation to give diphenylacetylene.(a) What is the structure, including stereochemistry, of the vinylic bromide?(b) If the sequence starts with (Z)-1,2-dibromo-1,2-diphenylethene, what is (are) the structure(s) of the intermediate dibromide(s)? What is the structure of the vinylic bromide?arrow_forwardCarbocations undergo 1,2-methyl shift or 1,2-hydride shift to produce a more stable intermediate. Give the major product(s) obtained from the reaction of the alkene with HBr.SHOW THE FORMATION OF THE CARBOCATION, THE SHIFT THAT MAY OCCUR, AND THE MAJOR PRODUCT FORMED.arrow_forwardButanone undergoes a nucleophilic addition with a Grignard reagent made from 1-bromopropane and magnesium metal in THF solution. The alkoxide formed from the nucleophilic addition is then conveted into the final product by the careful addition of dilute acid. Complete the mechanism by following the instructions provided for each step. Step 1. Nucleophilic Addition in THFarrow_forward
- The following are intermediate products in the stepwise synthesis of compound 1 from benzene. Give the correct sequence of reactions with the appropriate reagents that will lead to the correct intermediate products and final product.arrow_forward(a) Propose a mechanism for the conversion of cis-hex-3-ene to the epoxide (3,4-epoxyhexane)and the ring-opening reaction to give the glycol, hexane-3,4-diol. In your mechanism, payparticular attention to the stereochemistry of the intermediates and products.(b) Repeat part (a) for trans-hex-3-ene. Compare the products obtained from cis- andtrans-hex-3-ene. Is this reaction sequence stereospecific?arrow_forwardWittig reactions with the following -chloroethers can be used for the synthesis of aldehydes and ketones. (a) Draw the structure of the triphenylphosphonium salt and Wittig reagent formed from each chloroether. (b) Draw the structural formula of the product formed by treating each Wittig reagent with cyclopentanone. Note that the functional group is an enol ether or, alternatively, a vinyl ether. (c) Draw the structural formula of the product formed on acid-catalyzed hydrolysis of each enol ether from part (b).arrow_forward
- Following is a retrosynthetic scheme for the synthesis of the tricyclic diene on the left. Show how to accomplish this synthesis from 2-bromopropane, cyclopentadiene, and 2-cyclohexenone.arrow_forwardReaction of -pinene with borane followed by treatment of the resulting trialkylborane with alkaline hydrogen peroxide gives the following alcohol. Of the four possible cis,trans isomers, one is formed in over 85% yield. (a) Draw structural formulas for the four possible cis,trans isomers of the bicyclic alcohol. (b) Which is the structure of the isomer formed in 85% yield? How do you account for its formation? Create a model to help you make this prediction.arrow_forwardA standard method for the synthesis of ethers is an SN2 reaction between an alkoxide and an alkyl halide.Show possible combinations of alkoxide and alkyl halide for the preparation of the following ethers. Which of these can be prepared effectively by this method?arrow_forward
arrow_back_ios
arrow_forward_ios
Recommended textbooks for you

 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning


Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License