
Concept explainers
Thinking Mechanistically About
The preparation and properties of alkynes extend some topics explored in earlier chapters:
* Alkynes can be prepared by elimination reactions related to the
* Alkynes can be prepared by
* Alkynes undergo addition reactions, especially electrophilic addition, with many of the same compounds that add to alkenes.
The greater s character of sp hybrid orbitals compared with
* The
* Unlike alkenes, alkynes are reduced by metals, especially
* Unlike alkenes, alkynes can undergo nucleophilic as well as electrophilic addition.

Problems
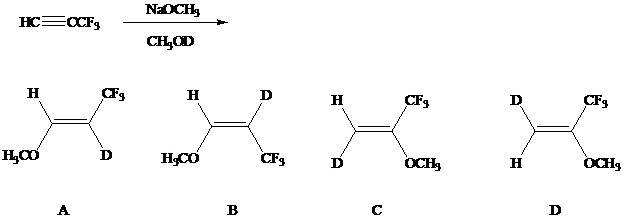
Nucleophilic addition can occur with alkynes that bear strong electron-attractingsubstituents such as
Want to see the full answer?
Check out a sample textbook solution
Chapter 9 Solutions
Loose Leaf Student Solutions Manual Organic Chemistry
- Following is a balanced equation for bromination of toluene. (a) Using the values for bond dissociation enthalpies given in Appendix 3, calculate H0 for this reaction. (b) Propose a pair of chain propagation steps and show that they add up to the observed reaction. (c) Calculate H0 for each chain propagation step. (d) Which propagation step is rate-determining?arrow_forwardWhen 2-methylpropane is monochlorinated in the presence of light at room temperature, 36% of the product is 2-chloro-2-methylpropane and 64% is 1-chloro-2-methylpropane. From these data, calculate how much easier it is to remove a hydrogen atom from a tertiary carbon than from a primary carbon under these conditions.arrow_forwardWhat would be the major product obtained from the reaction of Br2 with 1-butene if the reaction were carried out in the solvent dichloromethane? Draw the molecule.arrow_forward
- 1.- Why are carbon nanotubes and graphene currently considered as reinforcing additives for polymers? What effect do they have on the polymer? What is the mechanism by which they can enhance the Young’s modulus and tensile strength of a polymer? 2.- Kapton is the trade name of a polyimide a) How would you synthesize this polymer? b) During the first stage of the reaction, poly(amic acid) is formed. If you need to follow this stage of the polymerization, what spectroscopic techniques would you use and what groups should you focus on? c) How would you form Kapton films, which can be used as support for solar sails? d) What are the disadvantages of Kapton that have limited their use? Why does this polymer present this weakness?arrow_forwardbutene-1 acts on a combination of three reactants.Write the corresponding reactions. a) H2, Br2, N2 b) Na, NaOH, HCl c) H2O, HBr, KMnO4 d) Cl2, CO2, H2SO4arrow_forwardFunctional groups such as alkynes react the same in complex molecules as they do in simpler structures. The following example of alkyne reaction were taken from syntheses carried out in the research group of E. J. Corey at Harvard University. You can assume that the reactions listed involve only the alkyne, not any of the functional groups present in the molecules. Draw the expected products for the following reaction .arrow_forward
- For parts b and c could you please explain why there is a cis isomer formed and how to know when there will be one. For part e do you have to rearrange the compound to achieve the antiperiplanar orientation? Predict the products formed by sodium hydroxide-promoted dehydrohalogenation of the following compounds. In each case, predict which will be the major product. (b) 2-chlorobutane (c) 3-bromopentane (e) trans-1-bromo-2-methylcyclohexane.arrow_forwardDoes the data provide evidence for the claim that the reaction of Br2 with alkenes proceeds via anti‐addition? The mleting point of the product was found to be 234.5-235.5C chemical reaction: trans-stilbene+pyrimindine tribromide --->acetic acid 1,2-di bromo-1,2-diphenylethanearrow_forwardYou are required to synthesize 2-bromopentane from the reaction between an alkene with HBr. Which alkene, 1-pentene or 2-pentene, should you react with HBr in order to get 2-bromopentane? Give an explanation.arrow_forward
- Following is a balanced equation for the allylic bromination of propene. CH2==CHCH3 + Br2 h CH2==CHCH2Br + HBr (a) Calculate the heat of reaction, H 0, for this conversion. (b) Propose a pair of chain propagation steps and show that they add up to the observed stoichiometry. (c) Calculate the H 0 for each chain propagation step and show that they add up to the observed H 0 for the overall reaction.arrow_forwardA benzene ring alters the reactivity of a neighboring group in the so-called “benzylic” position, similarly to how a double bond alters the reactivity of groups in the “allylic” position. Benzylic cations, anions, and radicals are all more stable than simple alkyl intermediates. a) Use resonance structures to show the delocalization of the positive charge, negative charge, and unpaired electron of the benzyl cation, anion, and radical.arrow_forwardReaction of 2-methyl-2-butene (above) with HBr might, in principle, lead to a mixture of two alkyl bromide addition products. Draw these two alkyl bromides.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
