
ETEXT+MASTERINGCHEMISTRY STANDALONE AC
7th Edition
ISBN: 9781269736947
Author: Bruice
Publisher: PEARSON
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 52P
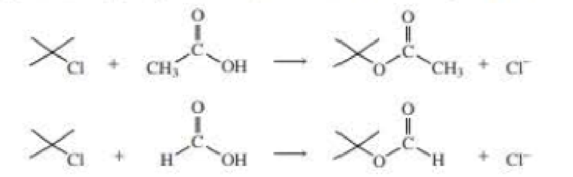
tert-Butyl chloride undergoes solvolysis in both acetic acid and formic acid. Solvolysis occurs 5000 times faster in one of these two solvents than in the other. In which solvent is solvolysis faster? Explain your answer. (Hint: Formic acid is more polar than acetic add.)
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Fill in the appropriate reagent or starting material in each of the following reactions.
Enolates are formed by deprotonation of an α-carbon hydrogen. Answer the following questions about enolate formation.
In the molecule shown, select the α-carbon hydrogen that would be removed to form an enolate when sodium hydroxide is used as a base. (use photo to answer this question).
Then, Draw the thermodynamic enolate that results for the molecule in Part 1. Draw only the enolate resonance form that includes a formal charge on the α carbon. Be sure to indicate that formal charge as well as any lone pair of electrons in your answer.
Enolates are formed by deprotonation of an α-carbon hydrogen. Answer the following questions about enolate formation.
In the molecule shown, select the α-carbon hydrogen that would be removed to form an enolate when NaOEt is used as a base.
Draw the thermodynamic enolate that results for the molecule in Part 1. Draw only the enolate resonance form that includes a formal charge on the α carbon. Be sure to indicate that formal charge as well as any lone pair of electrons in your answer.
Chapter 9 Solutions
ETEXT+MASTERINGCHEMISTRY STANDALONE AC
Ch. 9.1 - Prob. 3PCh. 9.1 - Does increasing the energy barrier for an SN2...Ch. 9.1 - Rank the following alkyl bromides from most...Ch. 9.2 - Prob. 8PCh. 9.2 - Prob. 9PCh. 9.2 - Prob. 10PCh. 9.2 - Prob. 11PCh. 9.2 - Which substitution reaction lakes place more...Ch. 9.2 - Prob. 14PCh. 9.2 - Prob. 16P
Ch. 9.3 - Prob. 17PCh. 9.4 - Prob. 18PCh. 9.5 - Prob. 19PCh. 9.5 - Prob. 20PCh. 9.5 - Prob. 21PCh. 9.5 - Prob. 22PCh. 9.6 - Prob. 23PCh. 9.6 - Prob. 24PCh. 9.6 - Which of the following reactions take place more...Ch. 9.7 - Prob. 26PCh. 9.7 - Prob. 27PCh. 9.7 - Prob. 28PCh. 9.7 - Prob. 30PCh. 9.7 - Under which of the following reaction conditions...Ch. 9.8 - After a proton is removed from the OH group, which...Ch. 9.8 - Prob. 33PCh. 9.9 - Prob. 34PCh. 9 - Prob. 1PCh. 9 - Methoxychlor is an insecticide that was intended...Ch. 9 - Prob. 35PCh. 9 - Prob. 36PCh. 9 - Prob. 37PCh. 9 - Prob. 38PCh. 9 - Prob. 39PCh. 9 - Prob. 40PCh. 9 - Starting with cyclohexene, how can the following...Ch. 9 - Prob. 42PCh. 9 - The pKa of acetic acid in water is 4.76. What...Ch. 9 - Prob. 44PCh. 9 - Prob. 45PCh. 9 - Prob. 46PCh. 9 - Prob. 47PCh. 9 - Prob. 48PCh. 9 - Prob. 49PCh. 9 - Prob. 50PCh. 9 - Prob. 51PCh. 9 - tert-Butyl chloride undergoes solvolysis in both...Ch. 9 - Prob. 53PCh. 9 - Prob. 54PCh. 9 - In which solventethanol or diethyl etherwould the...Ch. 9 - Prob. 56PCh. 9 - Two bromoethers are obtained from the reaction of...Ch. 9 - Prob. 58PCh. 9 - Prob. 59PCh. 9 - Prob. 60PCh. 9 - Propose a mechanism for the following reaction:Ch. 9 - Prob. 62PCh. 9 - Prob. 63PCh. 9 - Prob. 64PCh. 9 - Prob. 65PCh. 9 - When equivalent amounts of methyl bromide nod...Ch. 9 - Prob. 67PCh. 9 - The reaction of chloromethane with hydroxide ion...
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- How can we explain why, in base-catalyzed halogenation of acetone, the second (and third) halogenation occurs on the same carbon, but not the carbon in the other methyl group.arrow_forwardGive minor or major product?arrow_forwardWhile CH3OH is the solvent in the reaction, and a nucleophilic one, why doesn’t CH3OH act as the nucleophile in this reaction?arrow_forward
- Can you please help answer this problem and elaborate the reagents.arrow_forwardAlkyl halides 1-chlorobutane 2-chlorobutane Allyl chloride 2-chloro-2-methylpropane 1-chloro-2-methylpropane 2-bromobutane SN2 conditions = Sodium Iodide in Acetone SN1 conditions = Silver Nitrate in Ethanol 1. Which compound was reactive under both SN2 and SN1 reaction conditions? Explain why.arrow_forwardExplain using resonance structures of the intermediates (please explain and draw them out) why the bromination of phenol is faster than the bromination of phenyl ester?arrow_forward
- Rank the reactivity of the following compounds according to the SN2 reaction? Please explain why a) Methyl chloride b) Methyl iodide c) isopropyl chloridearrow_forwardThis is E2 Reaction, but can anyone explain why the major product is this in answer?arrow_forwardRank these in order of increasing reactivity in an SN1 reactionarrow_forward
- Give the major organic product for each step of the following reactionarrow_forwardAccording to the LUMO density map, on which face is the LUMO electronically more exposed to nucleophiles? Based on this data, which face would be more electronically favored? (These questions go hand in hand.)arrow_forwardAlcohols react with sulfonyl chlorides to form sulfonate esters. Only the O-H bond of the alcohol is broken in the reaction, and so no inversion of configuration occurs. The resulting sulfonate esters are reactive in SN1 and SN2 reactions since the sulfonate group is a very weak base and is therefore a good leaving group.Draw curved arrows to show the movement of electrons in this step of the mechanism.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:9780618974122
Author:Andrei Straumanis
Publisher:Cengage Learning
Alcohols, Ethers, and Epoxides: Crash Course Organic Chemistry #24; Author: Crash Course;https://www.youtube.com/watch?v=j04zMFwDeDU;License: Standard YouTube License, CC-BY