
Concept explainers
(a)
Interpretation:
The condensed electron configurations and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(a)
Answer to Problem 9.20P
The condensed electronic configuration of
The condensed electronic configuration of
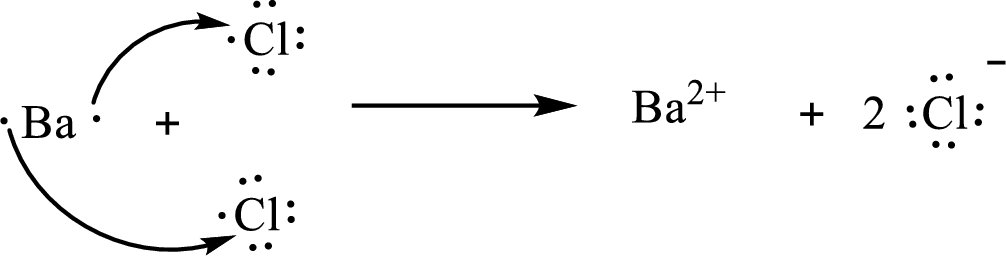
The Lewis orbital diagram is as follows:
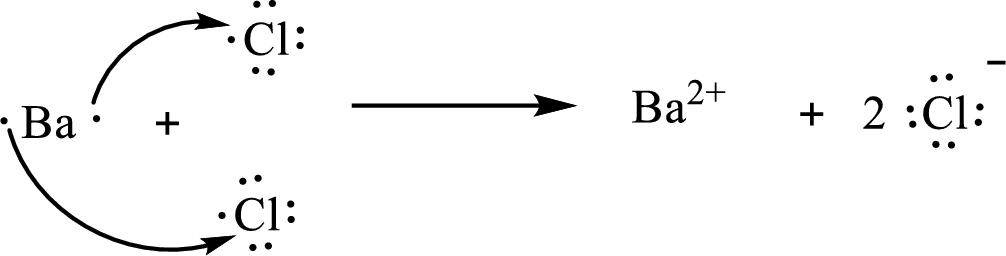
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of a barium atom
The condensed electronic configuration of the chlorine atom
Barium atom loses two electrons to form
The condensed electronic configuration of
The condensed electronic configuration of
Barium atom loses two electrons to form
The Lewis orbital diagram is as follows:
(b)
Interpretation:
The condensed electron configurations, partial orbital diagrams, and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is a pictorial representation of the electrons present in an orbital. Each orbital can occupy only two electrons of opposite spin.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(b)
Answer to Problem 9.20P
The condensed electronic configuration of
The condensed electronic configuration of
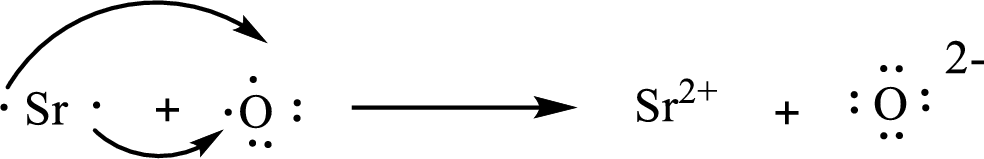
The Lewis orbital diagram is as follows:
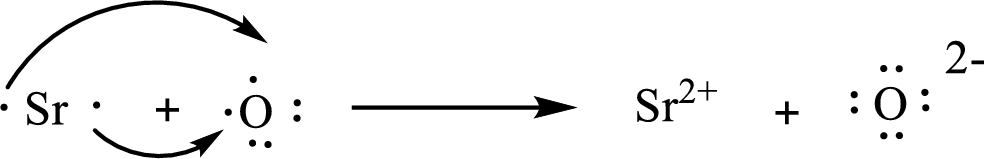
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of a strontium atom
The condensed electronic configuration of oxygen atom
Strontium atom loses two electrons to form
The condensed electronic configuration of
The condensed electronic configuration of
Strontium atom loses two electrons to form
The Lewis orbital diagram is as follows:
(c)
Interpretation:
The condensed electron configurations, partial orbital diagrams, and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is a pictorial representation of the electrons present in an orbital. Each orbital can occupy only two electrons of opposite spin.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(c)
Answer to Problem 9.20P
The condensed electronic configuration of
The condensed electronic configuration of
The Lewis orbital diagram is as follows:
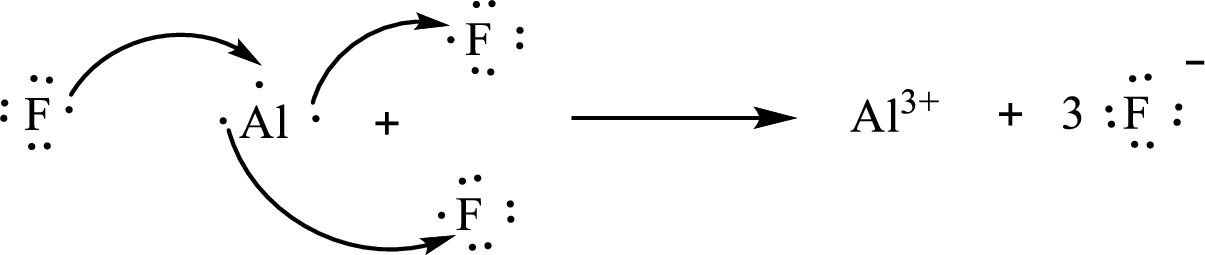
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of an aluminium atom
The condensed electronic configuration of the fluorine atom
Aluminium atom loses three electrons to form
The condensed electronic configuration of
The condensed electronic configuration of
Aluminium atom loses three electrons to form
The Lewis orbital diagram is as follows:
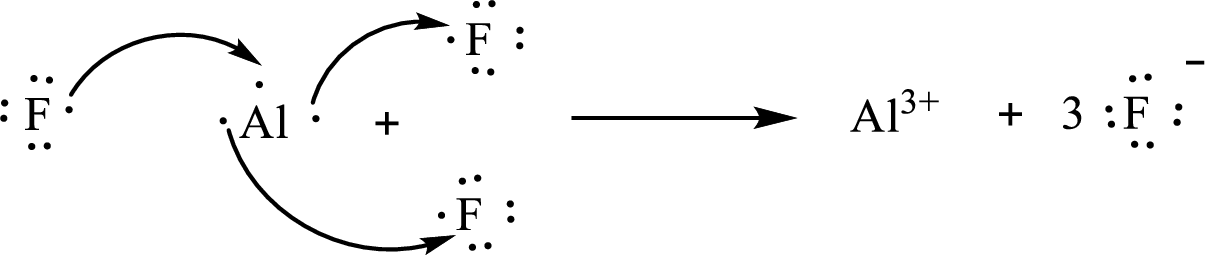
(d)
Interpretation:
The condensed electron configurations, partial orbital diagrams, and Lewis symbols to depict the formation of ions formed from atoms
Concept introduction:
The electronic configuration tells about the distribution of electrons in various atomic orbitals. The condensed electronic configuration is a way to write the electronic configuration where the inner shell configurations are compressed to the nearest noble gas configuration and only the valence shell configuration is written in the expanded form.
The partial orbital diagram is a pictorial representation of the electrons present in an orbital. Each orbital can occupy only two electrons of opposite spin.
Lewis electron-dot symbol is a representation employed to donate the valence electron present in the atom. It includes atom symbol to represent inner electrons and nucleus and the dots represent the valence present in the atom.
(d)
Answer to Problem 9.20P
The condensed electronic configuration of
The condensed electronic configuration of
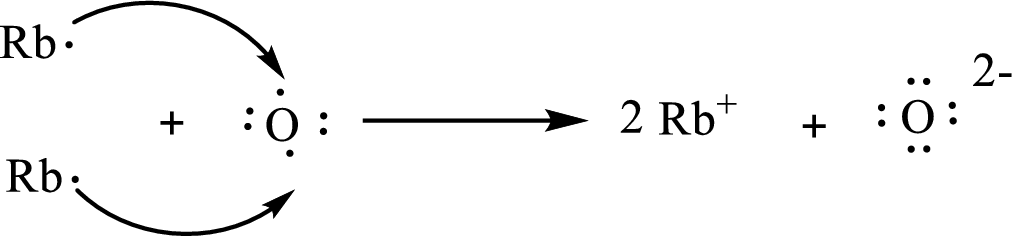
The Lewis orbital diagram is as follows:
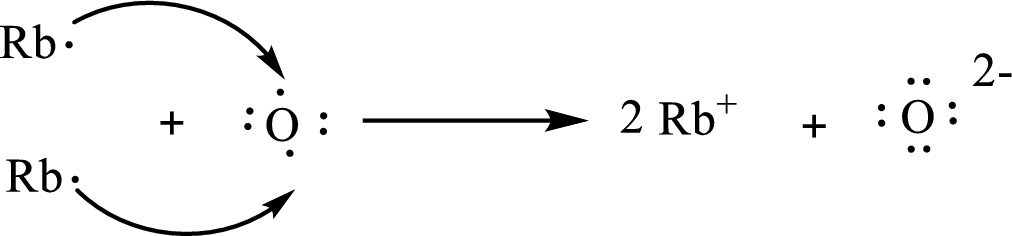
The formula of the compound formed is
Explanation of Solution
The condensed electronic configuration of a rubidium atom
The condensed electronic configuration of oxygen atom
Two rubidium atoms lose one electron respectively to form
The condensed electronic configuration of
The condensed electronic configuration of
Two rubidium atoms lose one electron respectively to form
The Lewis orbital diagram is as follows:
Want to see more full solutions like this?
Chapter 9 Solutions
GEN CMB CHEM; CNCT+;ALEKS 360
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY





