
Student Study Guide and Solutions Manual for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
8th Edition
ISBN: 9781305864504
Author: Brent L. Iverson, Sheila Iverson
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Concept explainers
Textbook Question
Chapter 9, Problem 9.39P
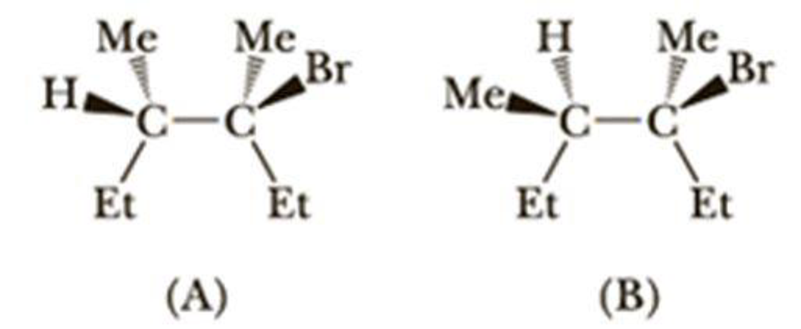
Following are diastereomers (A) and (B) of 3-bromo-3,4-dimethylhexane. On treatment with sodium ethoxide in ethanol, each gives 3,4-dimethyl-3-hexene as the major product. One diastereomer gives the E
Expert Solution & Answer
Trending nowThis is a popular solution!

Students have asked these similar questions
The optically active (2R)-2-phenyl-2-butanol reacts in hydrochloric acid to form haloalkanes and alkenes. The substitution reaction is reported to occur with 100% racemization.
Give the structures of the enantiomers that form during the substitution and indicate how much of each is formed.
Propose a mechanism for the substitution reaction to yield the R product.
What reagents would you use to achieve 100% retention of configuration.
Two geometric isomers are obtained during the elimination reaction. Explain mechanistically which alkene will be the main product.
5. Compound A, C 10H 18O, undergoes reaction with dilute H 2SO 4 at 50 °C to yield a mixture of two alkenes, C 10H 16. The major alkene B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Which of the following reactions are correct.
A certain compound of molecular formula C19H38 was isolated from fish oil and from plankton. On hydrogenation it gave 2,6,10,14-tetramethylpentadecane. Ozonolysis gave (CH3)2C O and a 16-carbon aldehyde. What is the structure of the natural product? What is the structure of the aldehyde?
Chapter 9 Solutions
Student Study Guide and Solutions Manual for Brown/Iverson/Anslyn/Foote's Organic Chemistry, 8th Edition
Ch. 9.1 - Prob. 9.1PCh. 9.3 - Prob. 9.2PCh. 9.3 - Prob. 9.3PCh. 9.3 - Prob. 9.4PCh. 9.4 - Prob. 9.5PCh. 9.5 - Predict the -elimination product(s) formed when...Ch. 9.7 - Prob. 9.7PCh. 9.9 - Predict whether each reaction proceeds...Ch. 9.9 - Prob. AQCh. 9.9 - Prob. BQ
Ch. 9.9 - Prob. CQCh. 9.9 - Prob. DQCh. 9.10 - Prob. 9.9PCh. 9 - Prob. 9.10PCh. 9 - Prob. 9.11PCh. 9 - Prob. 9.12PCh. 9 - Prob. 9.13PCh. 9 - Prob. 9.14PCh. 9 - Prob. 9.15PCh. 9 - Treatment of 1-aminoadamantane, C10H17N, with...Ch. 9 - Prob. 9.17PCh. 9 - Prob. 9.18PCh. 9 - Prob. 9.19PCh. 9 - Prob. 9.20PCh. 9 - Attempts to prepare optically active iodides by...Ch. 9 - Draw a structural formula for the product of each...Ch. 9 - Prob. 9.23PCh. 9 - Alkenyl halides such as vinyl bromide, CH2=CHBr,...Ch. 9 - Prob. 9.25PCh. 9 - Prob. 9.26PCh. 9 - Prob. 9.27PCh. 9 - Show how you might synthesize the following...Ch. 9 - Prob. 9.29PCh. 9 - 1-Chloro-2-butene undergoes hydrolysis in warm...Ch. 9 - Prob. 9.31PCh. 9 - Prob. 9.32PCh. 9 - Solvolysis of the following bicyclic compound in...Ch. 9 - Which compound in each set undergoes more rapid...Ch. 9 - Prob. 9.35PCh. 9 - Prob. 9.36PCh. 9 - Draw structural formulas for the alkene(s) formed...Ch. 9 - Prob. 9.38PCh. 9 - Following are diastereomers (A) and (B) of...Ch. 9 - Prob. 9.40PCh. 9 - Elimination of HBr from 2-bromonorbornane gives...Ch. 9 - Which isomer of 1-bromo-3-isopropylcyclohexane...Ch. 9 - Prob. 9.43PCh. 9 - Prob. 9.44PCh. 9 - Draw a structural formula for the major organic...Ch. 9 - When cis-4-chlorocyclohexanol is treated with...Ch. 9 - Prob. 9.47PCh. 9 - The Williamson ether synthesis involves treatment...Ch. 9 - The following ethers can, in principle, be...Ch. 9 - Prob. 9.50PCh. 9 - Prob. 9.51PCh. 9 - Prob. 9.52PCh. 9 - Prob. 9.53PCh. 9 - Prob. 9.54PCh. 9 - Write the products of the following sequences of...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Prob. 9.59PCh. 9 - Another important pattern in organic synthesis is...Ch. 9 - Using your reaction roadmap as a guide, show how...Ch. 9 - Prob. 9.62PCh. 9 - Prob. 9.63PCh. 9 - Prob. 9.64P
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- Compound A has molecular formula C4H10. Compound A gives two monochlorides, B and C, on photochemical chlorination. Treatment of either of these monochlorides with potassium tert-butoxide gives the same alkene (C4H8) as the product, but B leads to just one isomer of the alkene, D, whereas C gives D and another isomer of the alkene, E. Treatment of monochlorides B and C with aqueous ethanol gives products F and G, respectively, both of which are of molecular formula C4H100. What are the chemical names of compounds A-G?arrow_forwardFollowing are diastereomers (A) and (B) of 3-bromo-3,4-dimethylhexane. On treat- ment with sodium ethoxide in ethanol, each gives 3,4-dimethyl-3-hexene as the major product. One diastereomer gives the E alkene, and the other gives the Z alkene. Which diastereomer gives which alkene? Account for the stereoselectivity of each B-elimination. H C-C Mẹ Me Br H Ме Br C-C Me Et Et Et Et (A) (В)arrow_forwardFollowing are two diastereomers of 3-bromo-3,4-dimethylhexane. On treatment with sodium ethoxide in ethanol, each gives 3,4-dimethyl-3-hexene as the major product. One diastereomer gives the E alkene, and the other gives the Z alkene. Which diastereomer gives the Z alkene? Et Me Et Et Et Br Me H Ме b H Br Me aarrow_forward
- 2- Give the product for the addition of chlorine when HCl reacts with 3-Hexyne in acetic acid (CH3COOH). Show the correct stereochemistry, (is the product Z or E isomer). Identify the product in the following reaction: CH3CH₂CH₂C=CH + 2Cl₂ 3- What products are obtained by the hydration of the following Alkyne: CH3CH₂CH₂C=CCH₂CH₂CH3 1 ?arrow_forwardCompound A(C7H15Br) is not a primary alkyl bromide. It yields a single alkene(compound B) on being heated with sodium ethoxide in ethanol. Hydrogenation of compound B yields 2,4-dimethylpentane. Identify compounds A and B.arrow_forwardα-Terpinene, C10H16, is a pleasant-smelling hydrocarbon that has been isolated from oil of marjoram. On hydrogenation over a palladium catalyst, α-terpinene reacts with 2 molar equivalents of H2 to yield a hydrocarbon, C10H20. On ozonolysis, followed by reduction with zinc and acetic acid, α-terpinene yields two products, glyoxal and 6-methyl-2,5- heptanedione. (a) How many degrees of unsaturation does a-terpinene have? (b) How many double bonds and how many rings does it have? (c) Propose a structure for a-terpinene.arrow_forward
- When the alkene A was treated first with Hg(OAc)2 in MeOH and second with NaBH4 the product was the ether B. Using curved arrows please give the mechanism for the first step of (Hg(OAc)2 in MeOH) this reaction, including any regioselectivity or stereoselectivity. H3C 1. Hg(OAc)2, MeOH H3C O-CH3 =CH2 ✓ -CH3 H3C 2. NaBH H3C A Barrow_forwardCompound X has the molecular formula C7H14. Hydrogenation of compound X produces 2,4-dimethylpentane. Hydroboration-oxidation of compound X produces a racemic mixture of 2,4-dimethyl-1-pentanol. Predict the major product(s) obtained when compound X is treated with aqueous acid (H3O*). Draw all the substrates, reagents, and productsarrow_forwardAn optically active monoterpene (compound A) with molecular formula C10H18O undergoes catalytic hydrogenation to form an optically inactive compound with molecular formula C10H20O (compound B). When compound B is heated with acid, followed by reaction with O3 and then with dimethyl sulfide, one of the products obtained is 4-methylcyclohexanone. Give possible structures for compounds A and B.arrow_forward
- Bromine reacts with alkenes in methanol according to the equation when this reaction was carried out with 4-tert-butylcyclohexene, only one isomer was formed with the molecular formula C12H23BrO (80% yield). Which of the following is the structure more reasonable for this compound? Explain your reasoning through a corresponding mechanism.arrow_forwardCompound A (C7H11Br) is treated with magnesium in ether to give B (C7H11MgBr), which reacts violently with D2O to give 1-methylcyclohexene with a deuterium atom on the methyl group (C). Reaction of B with acetone (CH3COCH3) followed by hydrolysis gives D (C10H18O). Heating D with concentrated H2 SO4 gives E (C10 H16), which decolorizes two equivalents of Br2 to give F (C10H16 Br4). E undergoes hydrogenation with excess H2 and a Pt catalyst to give isobutylcyclohexane. Determine the structures of compounds A through F, and show your reasoning throughout.arrow_forwardName (including E/Z stereochemistry) the five alkenes that can produce 3-bromo-3-methylhexane on reaction with HBr. Draw the skeletal structure of each molecule.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:Cengage Learning
Coenzymes and cofactors; Author: CH15 SWAYAM Prabha IIT Madras;https://www.youtube.com/watch?v=bubY2Nm7hVM;License: Standard YouTube License, CC-BY
Aromaticity and Huckel's Rule; Author: Professor Dave Explains;https://www.youtube.com/watch?v=7-BguH4_WBQ;License: Standard Youtube License