
Concept explainers
(a)
Draw the
(a)
Answer to Problem 173RP
The
Explanation of Solution
Draw the
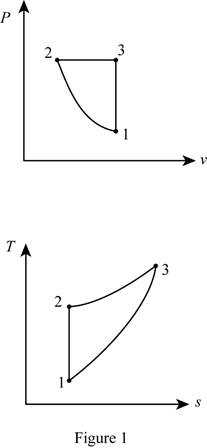
Thus, the
(b)
The expression for the back work ratio as a function of k and r.
(b)
Answer to Problem 173RP
The expression for the back work ratio as a function of k and r is
Explanation of Solution
Find the work of compression using the first law for process 1-2.
Here, heat interaction during the process 1-2 is
Write the expression of expansion work.
Here, gas constant is R, specific volume at state 2 and 3 is
Write the expression of back work ratio using the equations (I) and (II).
Here, temperature at state 1, 2, and 3 are
Conclusion:
Process 1-2: Isentropic
Calculate the ratio of
Here, pressure at state 1 and 2 is
Process 2-3: Constant pressure
Calculate the expression for
Here, volume at state 1 and 2 is
Process 3-1: Constant volume
Calculate the expression for
Substitute
Thus, the expression for the back work ratio as a function of k and r is
(c)
The expression for the cycle thermal efficiency as a function of k and r.
(c)
Answer to Problem 173RP
The expression for the cycle thermal efficiency as a function of k and r is
Explanation of Solution
Express out the heat addition and heat rejection in the process using first law to the closed system for processes 2-3 and 3-1.
Here, constant pressure specific heat is
Express the cycle thermal efficiency.
Conclusion:
Substitute
Substitute
Thus, the expression for the cycle thermal efficiency as a function of k and r is
(d)
The value of the back work ratio and thermal efficiency as r goes to unity.
(d)
Answer to Problem 173RP
The value of the back work ratio and thermal efficiency as r goes to unity is
Explanation of Solution
Recall the expression of back work ratio and apply the limits as r goes to infinity.
Thus, the value of the back work ratio as r goes to unity is
Recall the expression of cycle thermal efficiency and apply the limits as r goes to infinity.
Thus, the value of the thermal efficiency as r goes to unity is
From the results of cycle thermal efficiency and back work ratio values of 0 and 1, it shows that no expansion and net work can be done whether you add heat to the system when there is no compression
Want to see more full solutions like this?
Chapter 9 Solutions
EBK THERMODYNAMICS: AN ENGINEERING APPR
- An air-standard cycle is executed in a closed system with 0.5 kg of air and consists of the following three processes: 1-2 Isentropic compression from 100 kPa and 27C to 1 MPa 2-3 P = constant heat addition in the amount of 416 kJ 3-1 = 5 c1v + c2 heat rejection to initial state (c1 and c2 are constants) (a) Show the cycle on P-v and T-s diagrams. (b) Calculate the heat rejected. (c) Determine the thermal efficiency. Assume constant specific heats at room temperature.arrow_forwardA compression ratio of 8 is achieved by using an ideal air-standard Otto cycle engine. The working fluid has a pressure of 100 kPa and a temperature of 27°C at the start of the compression process, and the constant volume heat addition process supplies 800 kJ/kg of heat to the working fluid. What are the (a )the temperature, volume and pressure of the air at the end of each process (in K, m3 and kPa) (b) the net work output/cycle [kJ/kg], and (c) the thermal efficiency of this engine cyclearrow_forwardAn air-standard cycle is executed in a closed system and is composed of the following four processes: 1-2 Isentropic compression from 100 kPa and 27°C to 1 MPa 2-3 P = constant heat addition in amount of 2800 kJ/kg 3-4 V = constant heat rejection to 100 kPa P = constant heat rejection to initial state (a) Show the cycle on P-v and T-s diagrams. (b) Calculate the maximum temperature in the cycle. (c) Determine the thermal efficiency. Assume constant specific heats at room temperature.arrow_forward
- please use international valuesarrow_forward! Required information Problem 09.015 - 3-Step Air-Standard Cycle with Constant Specific Heats - DEPENDENT MULTI-PART PROBLEM - ASSIGN ALL PARTS An air-standard cycle with constant specific heats at room temperature is executed in a closed system with 0.003 kg of air and consists of the following three processes: 1-2 v= Constant heat addition from 95 kPa and 17°C to 380 kPa 2-3 Isentropic expansion to 95 kPa 3-1 P=Constant heat rejection to initial state The properties of air at room temperature are cp=1.005 kJ/kg-K, cv=0.718 kJ/kg-K, and k=1.4. Problem 09.015.a - P-v and T-s Diagrams for Constant Heat Capacity Air-Standard Cycle Show the cycle on P-v and T-s diagrams. (Please upload your response/solution using the controls provided below.) (You must provide an answer before moving on to the next part.) upload a response file (15MB max) Browse... No file selected. savearrow_forwardRequired information Problem 09.014 - 3-Step Air-Standard Cycle with Variable Specific Heats - DEPENDENT MULTI-PART PROBLEM - ASSIGN ALL PARTS An air-standard cycle with variable specific heats is executed in a closed system with 0.0025 kg of air and consists of the following three processes: 1-2 v= Constant heat addition from 95 kPa and 17°C to 380 kPa 2-3 Isentropic expansion to 95 kPa 3-1 P = Constant heat rejection to initial state Use data from tables. Problem 09.014.c - Cycle Efficiency for Variable Heat Capacity Air-Standard Cycle Determine the thermal efficiency. The thermal efficiency is %.arrow_forward
- An air-standard cycle, called the dual cycle, with constant specific heats is executed in a closed piston–cylinder system and is composed of the following five processes: 1-2 Isentropic compression with a compression ratio, r = V1/V2 2-3 Constant-volume heat addition with a pressure ratio, rp = P3/P2 3-4 Constant-pressure heat addition with a volume ratio, rc = V4/V3 4-5 Isentropic expansion while work is done until V5 = V1 5-1 Constant-volume heat rejection to the initial state. Sketch the P-v and T-s diagrams for this cycle.arrow_forwardA spark ignition engine that operates on Otto cycle has a compression ratio of 10. At the beginning of the compression process, air is at 100 kPa and 25 C. 750 kJ/kg of heat is transferred to air duringthe constant-volume heat-addition process. Consider the variation of specific heats with temperature. 1)Describe with the aid of diagrams the operational sequence of four stroke spark ignition engines 2)Explain the mechanical efficiency for ideal otto cycle of two and four stroke enginesarrow_forwardAn air-standard cycle with variable specific heats is executed in a closed system with 0.0045 kg of air and consists of the following three processes: 1–2 v = Constant heat addition from 95 kPa and 17°C to 380 kPa 2–3 Isentropic expansion to 95 kPa 3–1 P = Constant heat rejection to initial state Use data from tables. Calculate the thermal efficiency % ? Hint : The answer should be a percentagearrow_forward
- An air-standard cycle is executed within a closed piston–cylinder system, and it consists of the following three processes: 1–2 V = Constant heat addition from 100 kPa and 32°C to 850 kPa 2–3 Isothermal expansion until V3 = 8.5V2 3–1 P = Constant heat rejection to the initial state Assume air has constant properties with cv = 0.718 kJ/kg·K, cp = 1.005 kJ/kg·K, R = 0.287 kJ/kg·K, and k = 1.4. Determine the cycle thermal efficiency.arrow_forwardRequired information Problem 09.052 - Ideal Diesel Cycle with Variable Specific Heats - DEPENDENT MULTI-PART PROBLEM - ASSIGN ALL PARTS An air-standard Diesel cycle has a compression ratio of 16 and a cutoff ratio of 2. At the beginning of the compression process, air is at 103 kPa and 27°C. Account for the variation of specific heats with temperature. The gas constant of air is R= 0.287 kJ/kg-K. Problem 09.052.b - Thermal Efficiency in Variable Heat Capacity Diesel Cycle Determine the thermal efficiency. (You must provide an answer before moving on to the next part.) The thermal efficiency is 59.38 %.arrow_forwardAn air-standard cycle with variable specific heats is executed in a closed system with 0.003 kg of air, and it consists of the following three processes: 1-2 Isentropic compression from 3-1 v = constant heat rejection to initial state (a) Show the cycle on P-v and T-s diagrams. (b) Calculate the maximum temperature in the cycle. (c) Determine the thermal efficiency.arrow_forward
 Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press
Elements Of ElectromagneticsMechanical EngineeringISBN:9780190698614Author:Sadiku, Matthew N. O.Publisher:Oxford University Press Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON
Mechanics of Materials (10th Edition)Mechanical EngineeringISBN:9780134319650Author:Russell C. HibbelerPublisher:PEARSON Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education
Thermodynamics: An Engineering ApproachMechanical EngineeringISBN:9781259822674Author:Yunus A. Cengel Dr., Michael A. BolesPublisher:McGraw-Hill Education Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY
Control Systems EngineeringMechanical EngineeringISBN:9781118170519Author:Norman S. NisePublisher:WILEY Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning
Mechanics of Materials (MindTap Course List)Mechanical EngineeringISBN:9781337093347Author:Barry J. Goodno, James M. GerePublisher:Cengage Learning Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY
Engineering Mechanics: StaticsMechanical EngineeringISBN:9781118807330Author:James L. Meriam, L. G. Kraige, J. N. BoltonPublisher:WILEY





