
Concept explainers
(a)
Interpretation: The reason for the bromination to be much more regioselective than chlorination needs to be explained.
Concept Introduction : Hess's law states that when the reactants are converted to products the change of enthalpy (i.e. the heat of reaction at constant pressure) does not dependent on the pathway between the initial and final states.
Hess’s law states that enthalpy changes are additive. Thus, for a single reaction,
(a)
Answer to Problem 10E
The radical construction depends on the statistical factor and bromination reaction the reaction is endothermic and hence the transition state is nearer to the radical generated than the
The formation of radical depends on the stability of the radical and more selectivity is attained .
Explanation of Solution
Considering the bromination and chlorination of an alkane to check the
In the propagation process, the free radical of the reagent in step by step reacts with the
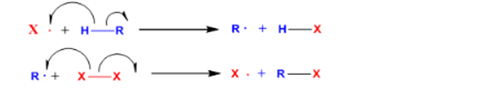
The initial stage controls the generation of a specific kind of radical.
The reaction enthalpy of this stage of bromination is measured as
The reaction enthalpy of this stage of chlorination is measured as follows:
Hence, the chlorination reaction is exothermic whereas the stage for bromination reaction is endothermic.
Hammond’s postulate the transition state of a reaction and would be nearer to the reactant in case of an exothermic reaction.
In chlorination reaction, the reaction is exothermic and thus the transition state is closer to the alkane than the radical generated.
The radical construction depends on the statistical factor.
In bromination reaction the reaction is endothermic and hence the transition state is nearer to the radical generated than the alkane.
The radical formation depends on the stability of the radical and more selectivity is attained.
(b)
Interpretation: The reason behind the dangerousness of fluorination needs to be explained.
Concept Introduction : Hess's law states that when the reactants are converted to products the change of enthalpy (i.e. the heat of reaction at constant pressure) does not dependent on the pathway between the initial and final states.
Hess’s law states that enthalpy changes are additive. Thus, for a single reaction,
(b)
Answer to Problem 10E
High exothermic nature of the bond formation, a huge amount of heat is liberated during fluorination hence the fluorination is dangerous.
Explanation of Solution
The bond formation enthalpy of carbon fluorine bond formation is as follows:
Due to this high exothermic nature of the bond formation, huge amount of heat is liberated during fluorination. Thus, the process is dangerous.
(C)
Interpretation: The reason behind the difficulty in the generation of an alkyl iodide by free radical chain halogenations needs to be explained.
Concept Introduction : Hess's law states that when the reactants are converted to products the change of enthalpy (i.e. the heat of reaction at constant pressure) does not dependent on the pathway between the initial and final states.
Hess’s law states that enthalpy changes are additive. Thus, for a single reaction,
(C)
Explanation of Solution
The iodination reaction of an alkane can be expressed as follows
The enthalpy change in this reaction is calculated as
As the reaction is endothermic in nature the reaction is difficult to carry out at room temperature.
The reverse reaction can also occur which further decreases the yield of the reaction.
Want to see more full solutions like this?
Chapter 9 Solutions
EBK EXPERIMENTAL ORGANIC CHEMISTRY: A M
- Rank the following dienes from most reactive to least reactive in a Diels Alder reaction. please explain stepsarrow_forwardWith respect to the CIP rules, assign the groups high or low priority - also what is the overall stereochemistry of the double bond?arrow_forwardExplain why free-radical halogenation usually gives mixtures of productsarrow_forward
- Explain why free-radical halogenation usually gives mixture of products.arrow_forwardWhat happens to the stereochemistry during and SN2 reaction? Why? Provide a reaction to illustrate this.arrow_forwardHow many monochlorinated products (constitutional isomers only) are possible for the structure below after radical chlorination?arrow_forward
- Please help with these Diels Alder reactions. Explain briefly!arrow_forwardWhat type of reaction is a Grignard reaction? What is the critical intermediate (which can be isolated and enables the final product)? Please include the NAME of the critical intermediate and explaination to why it is a certain reaction (eg. substition, addition). Thank you!arrow_forwardWe are doinga synthesis of vicinal dihalides by brominating alkenes. We are using silver nitrate test to characterize vicinal dihalides.arrow_forward
- a. What is the major monobromination product of the following reaction? Disregard stereoisomers. b. What is the anticipated percent yield of the major product (as a percentage of all the monobrominated products)?arrow_forwardthis is not graded quesiton; the reactions are all covered in Organic Chemistry, 6th Edition by Marc Loudon and Jim Parise. Chapter 15-17; why is this violating the term of use??? i dont't understand. this is not a graded quesitonarrow_forwarda) Predict the major product of the reaction and identify the type of reaction (substitution or addition). Explain the function of Pt used in the reaction. (with First image) b) Write the reaction of the mononitration of chlorobenzene and predict the major products with reasons. c) Identify all the carbon atoms in the following compounds that are sp2 (Circle the carbon(s) your selected on image two)arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
