
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
4th Edition
ISBN: 9781119776741
Author: Klein
Publisher: WILEY CONS
expand_more
expand_more
format_list_bulleted
Concept explainers
Question
Chapter 9.3, Problem 2LTS
Interpretation Introduction
Interpretation:
It is necessary to determine whether sodium acetate
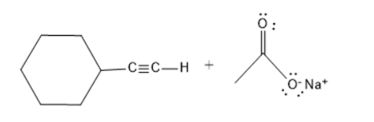
Concept Introduction :
The amount of hydrogen ions that one acid molecule can produce at most is one indicator of an acid's basicity. Due to its capacity to shed one proton or hydrogen atom, acetic acid is monobasic in nature.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Students have asked these similar questions
Please Could your help me for these questions 2, 3, 6. My reagents were 1-bromobutane and acetone
What products are formed when the compound below are heated strongly in presence of an appropriate solvent? use curved arrows to show movement of electrons
1.) Can you draw this out in more detail and explain the FC alkylation
2.) I do not understand what it is asking
3.) Can you confirm if it is correct or not
Chapter 9 Solutions
EBK ORGANIC CHEMISTRY-PRINT COMPANION (
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- in the electrophilic addition reactions exemplified in Scheme 1, Br2 is the a) nucleophile b) electrophile c) acid d) base e) arenearrow_forwardWhat is the most likely product functional group for the reaction below?Please include the letter with the answer. a. ester b. carboxylic acid c. alkene d. amide e. alcohol f. aldehyde g. ketonearrow_forwardWhich reagent is required to complete the reaction in Figure 11? [Generate a SMILES notation: https://jsme-editor.github.io/dist/JSME_test.html. Copy the notation (CTRL+C) and paste it as the answer to this question (CTRL+V).] * Your answerarrow_forward
- 1. Esters can be synthesized from the nucleophilic substitution reaction of anhydrides and alcohol in the presence of a base give a complete mechanism and the final product for the reaction below 2. using appropriate illustrations, explain what is meant by the tetrahedral transition state( also indicate the geometry for the starting material and product).arrow_forwardI know there is: 1. Carboxylic group 2. Ester. 3. Ortho-disubstituted phenyl. But my answer is still partial.arrow_forwardDraw the structural formula of the product of the reaction shown below. Please draw a clear and detailed image.arrow_forward
- Illustrated below is the hydrolysis of a C-C bond (in the first molecule) hydrolyzed by water (2nd molecule). Which pair of open boxes best identifies the location of the (-H) and (-OH) components of water on the products. Choose one from the following: (a) A (b) B (c) C (d) Darrow_forwardTrue or False : Constituent of turpentine, bicyclic terpene 3-carene, has 2 degrees of unsaturation (HDI = 2). Could you explain how to do this?arrow_forwarda) Write out the 3-step arrow pushing mechanism showing how 1-pentene is hydrated to make 2-pentanol. b) Draw the other 2 alkenes(don’t forget cis/trans isomers!) that could also be hydrated to make 2-pentanol. Briefly explain why1-pentene is the best choice.arrow_forward
- Will you please check my answer for the following ochem question ... We had to provide a stepwise synthesis for (CH3)3CCOCH(COCH2CH3)CO2H by using either ethyl acetoacetate or malonic ester synthesis and provide the bond line structures for the major organic products obtained at each step of the synthesis while also using the correct arrows to show flow of electrons.arrow_forwardRearrangements can occur during the dehydration of 1° alcohols even though no 1° carbocation is formed—that is, a 1,2-shift occurs as the C— OH2+ bond is broken, forming a more stable 2° or 3° carbocation, as shown in Equation [1]. Using this information, draw a stepwise mechanism for the reaction shown in Equation [2]. We will see another example of this type of rearrangement in Section 16.5C.arrow_forwardNeed help in drawing an electron pushing mechanism for the anti-Markovnikov hydrobromination reaction below along with all the necessary intermediates.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education
ChemistryChemistryISBN:9781259911156Author:Raymond Chang Dr., Jason Overby ProfessorPublisher:McGraw-Hill Education Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning
Principles of Instrumental AnalysisChemistryISBN:9781305577213Author:Douglas A. Skoog, F. James Holler, Stanley R. CrouchPublisher:Cengage Learning Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education
Organic ChemistryChemistryISBN:9780078021558Author:Janice Gorzynski Smith Dr.Publisher:McGraw-Hill Education Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning
Chemistry: Principles and ReactionsChemistryISBN:9781305079373Author:William L. Masterton, Cecile N. HurleyPublisher:Cengage Learning Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY
Elementary Principles of Chemical Processes, Bind...ChemistryISBN:9781118431221Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. BullardPublisher:WILEY

Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry
Chemistry
ISBN:9781259911156
Author:Raymond Chang Dr., Jason Overby Professor
Publisher:McGraw-Hill Education

Principles of Instrumental Analysis
Chemistry
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Cengage Learning

Organic Chemistry
Chemistry
ISBN:9780078021558
Author:Janice Gorzynski Smith Dr.
Publisher:McGraw-Hill Education

Chemistry: Principles and Reactions
Chemistry
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:Cengage Learning

Elementary Principles of Chemical Processes, Bind...
Chemistry
ISBN:9781118431221
Author:Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:WILEY