
Concept explainers
(a)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
Explanation of Solution
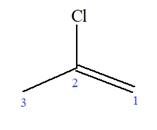
The given molecule is
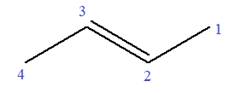
In this molecule, the root is propene. Thus, the longest carbon chain must have three carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the chain. The position of the double bond in the chain is between carbon atoms C1 and C2.
The root can be shown as:
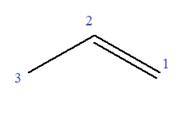
At C2 carbon atom of the root, one chlorine is attached. Thus, the structure of
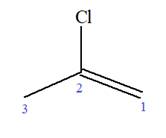
The structure of
(b)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
Explanation of Solution
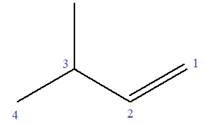
The given molecule is
In this molecule, the root is butene. Thus, the longest carbon chain must have four carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the chain. The position of the double bond in the chain is between carbon atoms C1 and C2.
The root can be shown as:
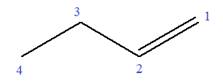
At C3 carbon atom of the root, a methyl substituent is attached.
Thus, the structure of

The structure of
(c)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
Explanation of Solution
The given molecule is
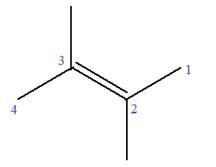
In this molecule, the root is butene. Thus, the longest carbon chain must have four carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the chain. The position of the double bond in the chain is between carbon atoms C2 and C3.
The root can be shown as:
At C2 and C3 carbon atoms of the root, two methyl substituents are attached.
Thus, the structure of
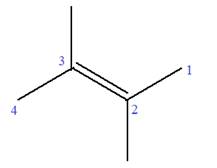
The structure of
(d)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents, are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
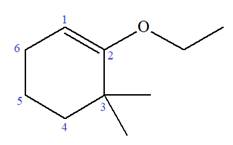
Explanation of Solution
The given molecule is
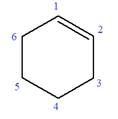
In this molecule, the root is cyclohexene. Thus, the largest carbon ring must have six carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the ring. The position of the double bond in the ring is always between carbon atoms C1 and C2.
The root can be shown as:
At C2 and C3 carbon atoms of the ring, one ethoxy and two methyl substituents are attached respectively. Thus, the structure of

The structure of
(e)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
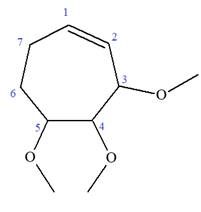
Explanation of Solution
The given molecule is
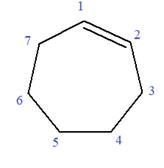
In this molecule, the root is cyclohexene. Thus, the largest carbon ring must have seven carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the ring. The position of the double bond in the ring is always between carbon atoms C1 and C2.
The root can be shown as:
At C2, C3, and C4 carbon atoms of this ring, three methoxy substituents are attached.
Thus, the structure of
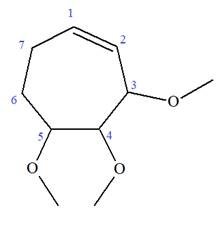
The structure of
(f)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
Explanation of Solution
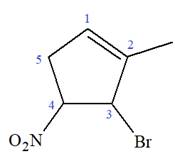
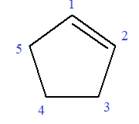
The given molecule is
In this molecule, the root is cyclohexene. Thus, the largest carbon ring must have six carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the ring. The position of the double bond in the ring is always between carbon atoms C1 and C2.
The root can be shown as:
At C2, C3, and C4 carbon atoms, bromine, methyl, and nitro group are attached.
Thus, the structure of
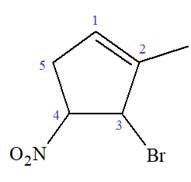
The structure of
(g)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
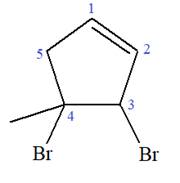
Explanation of Solution
The given molecule is
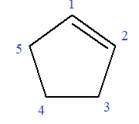
In this molecule, the root is cyclopentene. Thus, the largest carbon ring must have five carbon atoms. The suffix ‘ene’ indicates that there is a double bond in the ring. The position of the double bond in the ring is always between carbon atoms C1 and C2.
The root can be shown as:
At C3 and C4 carbon atoms of the ring, two bromine atoms and one methyl group are attached.
Thus, the structure of
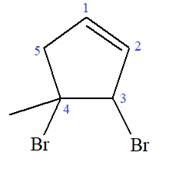
The structure of
(h)
Interpretation:
The structure for
Concept introduction:
In case of molecules containing a
If the root is a chain, numbering begins from that end of the chain which encounters the
The carbon atoms having a double or triple bond between them are always assigned C1 and C2, if the root is a ring. This must be done such that the locator numbers for the substituents are minimized. The lower of the two locator numbers for the
Answer to Problem B.26P
The structure for
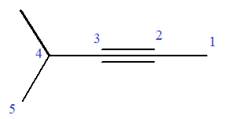
Explanation of Solution
The given molecule is
In this molecule, the root is pentyne. Thus, the longest carbon chain must have five carbon atoms. The suffix ‘yne’ indicates that there is a triple bond in the chain. The position of the triple bond in the chain is always between carbon atoms C2 and C3.
The root can be shown as:
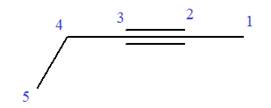
At C4 carbon atom of the root, a methyl group is attached.
Thus, the structure of
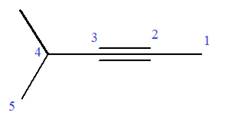
The structure of
Want to see more full solutions like this?
Chapter B Solutions
ORGANIC CHEMISTRY E-BOOK W/SMARTWORK5
- I would like to see the steps (arrows) that was taking to get all five isomers that is currently displayedbl separately and with all their steps . Chemical Reactions and Mechanisms. Dehydration of 4-methyl-2-pentanolarrow_forwarddraw the following (put in all H's): cyclobutenearrow_forwardcan you tell me what type of isomer are these and draw them as well? thanksarrow_forward
- Knowing that cyclohexane and hexene are isomers, suggest 2 methods allowing their separation.arrow_forward2)Major organic products? please write them both in their systematic IUPAC Please answer very soon will give rating surelyarrow_forwardDraw the structures of all monobromo derivatives of 2-methylpentane, C6H13Br, which have the bromine on a primary carbon.arrow_forward
- For the 1,2-dichlorocyclohexane stereoisomers, which conformation of the trans stereoisomer has the lower energy, the diaxial or the diequatorial conformer? Which isomer and conformation of the three you built has the lowest energy? E of all the isomers of 1,2-dichlorocyclohexane. Explain the differences in energy, i.e., identify the sources of strain in the conformations you built. Show the sources of strain in a drawing.arrow_forwardFor which double bonds are stereoisomers possible? (See attached)arrow_forwardGive two (2) examples of reactions for each of the types of organic reactions: substitution, elimination, addition and rearrangement. Show: a. the overall reaction (reactants --> products) b. the reaction mechanism (indicate intermediate product) c. indicate which is the reactive species or intermediate in the reaction (radical? electrophile? nucleophile?) d. overall description of the reaction eg., radical substitution or SR Include the names of the reactions.arrow_forward
 Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning
Organic Chemistry: A Guided InquiryChemistryISBN:9780618974122Author:Andrei StraumanisPublisher:Cengage Learning Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning
Chemistry: Principles and PracticeChemistryISBN:9780534420123Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward MercerPublisher:Cengage Learning


