
Concept explainers
The remaining steps in the industrial synthesis of vitamin A (as an acetate) are as follows: the Grignard reagent C from Problem 11 is allowed to react with the
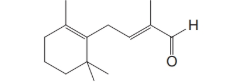
After acidification, the product obtained from this step is a
Want to see the full answer?
Check out a sample textbook solution
Chapter SRP Solutions
CHEM 313:ORG.CHEM V1 W/WLYLS BLKBRD >B
Additional Science Textbook Solutions
Chemistry & Chemical Reactivity
Chemistry: An Introduction to General, Organic, and Biological Chemistry (12th Edition) - Standalone book
Inorganic Chemistry
Chemistry: A Molecular Approach (4th Edition)
- 5. Compound A, C 10H 18O, undergoes reaction with dilute H 2SO 4 at 50 °C to yield a mixture of two alkenes, C 10H 16. The major alkene B, gives only cyclopentanone after ozone treatment followed by reduction with zinc in acetic acid. Which of the following reactions are correct.arrow_forwardAn unknown hydrocarbon A with the formula C6H10 reacts with 1 molar equivalent of H2 over a palladium catalyst to give B C6H12 (Rxn 1). Hydrocarbon A also reacts with OsO4 to give the glycol C (Rxn 2). A gives 5-oxohexanal on ozonolysis (Rxn 3). Draw the structures of A, B, and C. Give the reactions.arrow_forwardNitromethane is reacted with ethyl prop-2-enoate with EtO-Na+, EtOH 3 equivalents to give the product X(C16). X then reacts with H2/Raney Ni to give the product Y(C14), which in turn reacts with Na to give Z (C12). Indicate which products X, Y and Z are.arrow_forward
- Carototoxin is a natural pesticide produced by carrots, with formula C₁H2O. It undergoes hydrogenation with Pd to: give product A, C,H,O, and with Lindlar's catalyst to give product B, C,H2O. Ozonolysis followed by zinc leads to a mixture of methanal, octanal, 1,2-ethanedioic acid, 3-oxopropanoic acid, and 2-hydroxy-3-oxopropanoic acid. Draw possible structures for carototoxin, A, and B. Product B structure H2, Lindlar's Structure of carototoxin H2, Pd/C Product A structurearrow_forwardA graduate student was studying enzymatic reductions of cyclohexanones when she encountered some interesting chemistry. When she used an enzyme and NADPH to reduce the following ketone, she was surprised to find that the product was optically active. She carefully repurified the product so that no enzyme, NADPH, or other contaminants were present. Still, the product was optically active. If this reaction could be accomplished using H2 and a nickel catalyst, would the product be optically active? Explain.arrow_forwardAmines are converted into alkenes by a two-step process called Hofmann elimination. SN2 reaction of the amine with an excess of CH3I in the first step yields an intermediate that undergoes E2 reaction when treated with silver oxide as base. Pentylamine, for example, yields 1-pentene. Propose a structure for the intermediate, and explain why it readily undergoes elimination.arrow_forward
- When 2-pentene is treated with Cl2 in methanol, three products are formed. Account for the formation of each product (you need not explain their relative percentages).arrow_forwardCompound A and compound B both have molecular formula C6H12. Both compounds produce epoxides when treated with a peroxy acid (RCO3H). The epoxide resulting from compound A was treated with aqueous acid (H3O+) and the resulting diol has no chiral centers. Identify the two possible structures for compound A (enter two numbers separated by commas with no spaces, e.g. "1,2") The epoxide resulting from compound B was treated with (H3O*) and the resulting diol was a meso compound. Identify compound B 2 3 potn 7 1 5 6 4 8arrow_forwardComparing Hydration Products Using Two Different Methods Draw the product formed when CH3CH2C=CH is treated with each of the following sets of reagents: (a) H2O, H2SO4, HgSO4; and (b) R2BH, followed by H2O2, HO−.arrow_forward
- Give the products, if any, of the benzene + 2 CH3Cl + AlCl3 reaction:arrow_forwardCompound Y has molecular formula C,H12. Hydrogenation of compound Y produces methylcyclohexane. Treatment of compound Y with HBr in the presence of peroxides produces the following compound: Br Draw the product(s) when compound Y undergoes ozonolysis.arrow_forwardCompound AA has a molecular formula of C3H6O and gives a positiveresult using Tollen’s reagent. The reaction of compound AA with hotacidified potassium permanganate, KMnO4 gives compound BB. Thecatalytic hydrogenation of compound AA with nickel, Ni producedcompound CC. The reaction of compound BB with ethanamine,CH3CH2NH2 produces compound DD I) Draw the structural formula of compounds AA, BB, CC and DD. 2)Name the type of chemical reaction for the formation of compound CC.arrow_forward
 Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
Organic ChemistryChemistryISBN:9781305580350Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. FootePublisher:Cengage Learning
 EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT
EBK A SMALL SCALE APPROACH TO ORGANIC LChemistryISBN:9781305446021Author:LampmanPublisher:CENGAGE LEARNING - CONSIGNMENT


